Figure 6.
Recognition of partially assembled CCT subunits by the ZNRD2-HERC2 complex
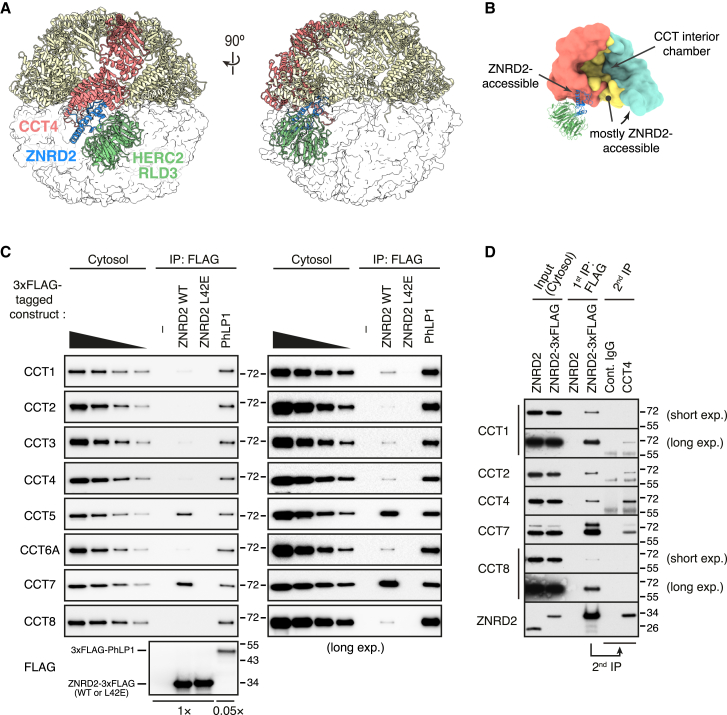
(A) The CCT4-ZNRD2-RLD3 model was docked into the complete CCT complex structure (PDB: 7lum) by aligning the CCT4 chains. The CCT4-ZNRD2-RLD3 model is colored, as in Figure 5A. The non-CCT4 subunits within the top ring of the CCT complex are shown as cartoons in khaki. The CCT subunits of the bottom ring are shown in transparent surface representation.
(B) Cartoon depicting a hypothetical partial CCT assembly comprising three subunits (colored in light coral, yellow, and cyan). One of the end subunits is compatible with binding the ZNRD2-HERC2 complex. The interior chamber is indicated for reference.
(C) HEK293T cells transiently overexpressing the indicated 3xFLAG-tagged proteins were subjected to non-denaturing anti-FLAG IP. The bound proteins were eluted with 3xFLAG peptide and subsequently analyzed by immunoblotting for the indicated proteins relative to serial 2-fold dilutions of the cytosolic fraction (cytosol) prepared from control transfected cells. 20-fold less of the 3xFLAG-PhLP1 sample was loaded on the gel relative to the control and ZNRD2-3xFLAG (WT and L42E) samples. Two exposures of the blots are shown for all CCT subunits.
(D) ZNRD2-KO cells stably overexpressing ZNRD2-3xFLAG (or untagged ZNRD2 as a negative control) were subjected to non-denaturing anti-FLAG IP. The bound proteins were eluted with 3xFLAG peptide and subjected to a second round of non-denaturing IP with anti-CCT4 antibody or control IgG. Samples were analyzed by immunoblotting as indicated.
See also Figure S7.