Figure S4.
Recognition of unassembled chaperonin subunits via the adaptor ZNRD2, related to Figure 3
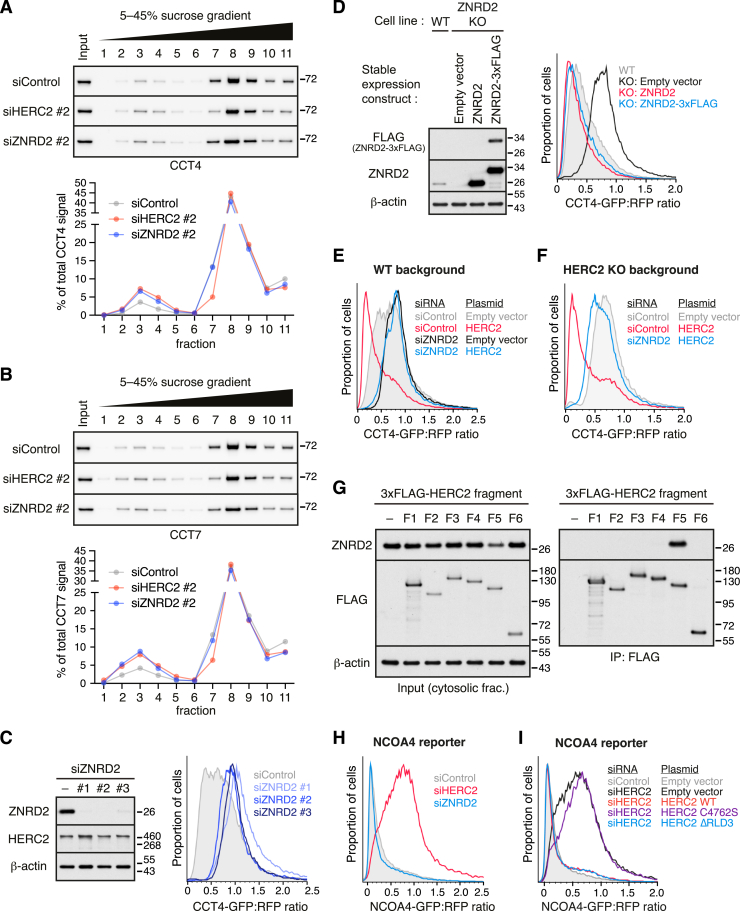
(A and B) Cytosolic extracts (input) from HEK293T cells treated for 5 days with the indicated siRNAs were separated into 11 fractions on a 5%–45% sucrose gradient. Input and gradient fractions were analyzed by immunoblotting for CCT4 (A) and CCT7 (B), and the signal intensities of the bands in each fraction were measured by densitometry. The graphs below the blots depict the distributions of CCT4 and CCT7 across the gradient. The proportion of unassembled CCT4 and CCT7 migrating in fractions 2–4 was used for the quantification shown in Figure 3B. Assembled CCT migrates primarily in fractions 7–9.
(C) Cells treated with control or ZNRD2-targeting siRNAs were transfected with the CCT4 reporter and analyzed by immunoblotting (left) and flow cytometry (right).
(D) WT Flp-In T-REx 293 cells and ZNRD2-KO cells with stably integrated empty vector, untagged ZNRD2, or ZNRD2-3xFLAG were transfected with the CCT4 reporter. Cells were analyzed by flow cytometry (right), and total cell lysates were analyzed by immunoblotting (left).
(E) HEK293T cells were treated with control or ZNRD2-targeting siRNAs, transfected with the CCT4 reporter and either empty vector or 3xFLAG-HERC2, and analyzed by flow cytometry.
(F) HERC2-KO Flp-In T-REx 293 cells were treated with control or ZNRD2-targeting siRNAs, transfected with the CCT4 reporter and either empty vector or 3xFLAG-HERC2, and analyzed by flow cytometry.
(G) The indicated HERC2 fragments with an N-terminal 3xFLAG tag (F1–F6) were transiently expressed in HEK293T cells and immunoprecipitated under non-denaturing conditions with anti-FLAG affinity resin (IP). Input (left) and IP samples (right) were analyzed by immunoblotting.
(H) A dual-color NCOA4 reporter (as in Figure S1A) was assessed for degradation by flow cytometry in HEK293T cells knocked down for HERC2 or ZNRD2. NCOA4 degradation, which is normally very efficient as indicated by the low GFP:RFP ratio, was inhibited by HERC2 knockdown but not ZNRD2 knockdown.
(I) HEK293T cells treated with control or HERC2-targeting siRNAs were transfected with the NCOA4 reporter together with empty vector or the indicated 3xFLAG-HERC2 constructs. In HERC2-knockdown cells, re-expression of HERC2 WT and ΔRLD3, but not catalytically inactive C4762S mutant, restored NCOA4 degradation.