Figure S5.
Additional characterization of in vitro ubiquitination assays, related to Figure 4
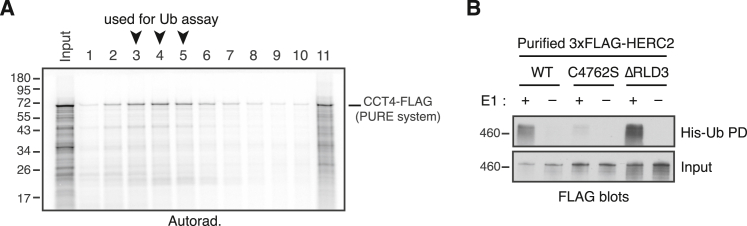
(A) 35S-methionine-labeled CCT4-3xFLAG was translated in a completely purified translation system (the PURE system; see method details) consisting of E. coli translation factors. The translation reaction was separated on a 5%–25% sucrose gradient and analyzed by SDS-PAGE and autoradiography. Soluble CCT4-3xFLAG migrating in fractions 3–5 (arrowheads) was recovered and used as the substrate for in vitro ubiquitination assays shown in Figure 4. Under the centrifugation conditions used, these fractions of the gradient would correspond to monomeric CCT4. A population of CCT4 fails to fold and is aggregated (fraction 11).
(B) Purified WT 3xFLAG-HERC2 and its mutants (C4762S and ΔRLD3) were tested for auto-ubiquitination by incubation with E1, E2 (UBCH5), His-Ub, and ATP, as indicated. The reactions were analyzed by anti-FLAG immunoblotting, either directly (input; bottom) or after His-Ub pull-down under denaturing conditions (His-Ub PD; top). Auto-ubiquitination was observed for HERC2 WT and ΔRLD3, but not for catalytically inactive C4762S mutant. As expected, ubiquitination was dependent on E1 enzyme.