Figure 4.
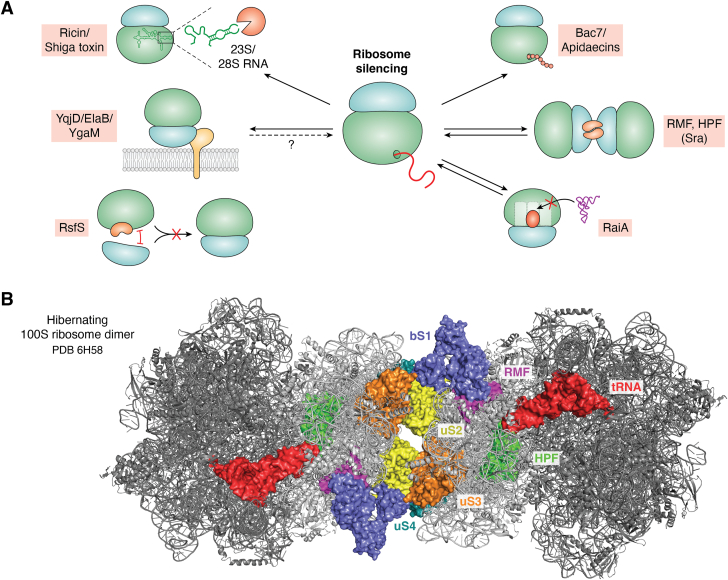
Ribosome silencing as stress response and defense strategy.A, RNA glycosidases, such as Ricin or Shiga toxin irreversibly inactivate ribosomes by cleaving the essential sarcin–ricin loop in 23S or 28S rRNA. Antimicrobial peptides (AMPs) such as Bac7 or apidaecins are secreted by eukaryotic cells and insert into the ribosomal peptide tunnel, leading to ribosome inactivation. Ribosome inactivation by AMPs or Ricin-like enzymes mainly serves as defense mechanism against pathogens and predators. Reversible ribosome inactivation is achieved by multiple proteins, which are expressed in response to stress or starvation conditions. Ribosomes are inactivated by dimerization via the coordinated activity of the ribosome modulation factor (RMF) and the hibernation promoting factor (HPF). The activity of RMF is potentially supported by the stationary phase–induced ribosome-associated protein (Sra). RaiA corresponds to a HPF homologue that binds to the P-site of the peptidyl-transferase domain and prevents the access of tRNAs. Yet another mechanism is employed by RsfS, which acts as an anti-association factor and prevents the 50S subunit from joining the 30S subunit. Finally, the three paralogs YqjD, ElaB, and YgaM are so far the only known membrane-bound proteins that have been implicated in ribosome hibernation. However, their mode of action is so far unknown. B, cryo-EM structure of the hibernating 100S ribosome dimer (PDB 6H58). The 30S and 50S subunits are colored in light gray and dark gray, respectively. The binding positions of RMF (magenta), HPF (green), and the tRNA (red) in the E-site of the peptidyltransferase domain are indicated. The localization of the 30S ribosomal proteins, which are involved in the ribosomal contacts, is indicated (bS1 [blue], uS2 [yellow], uS3 [bright orange], and uS4 [deep teal]).