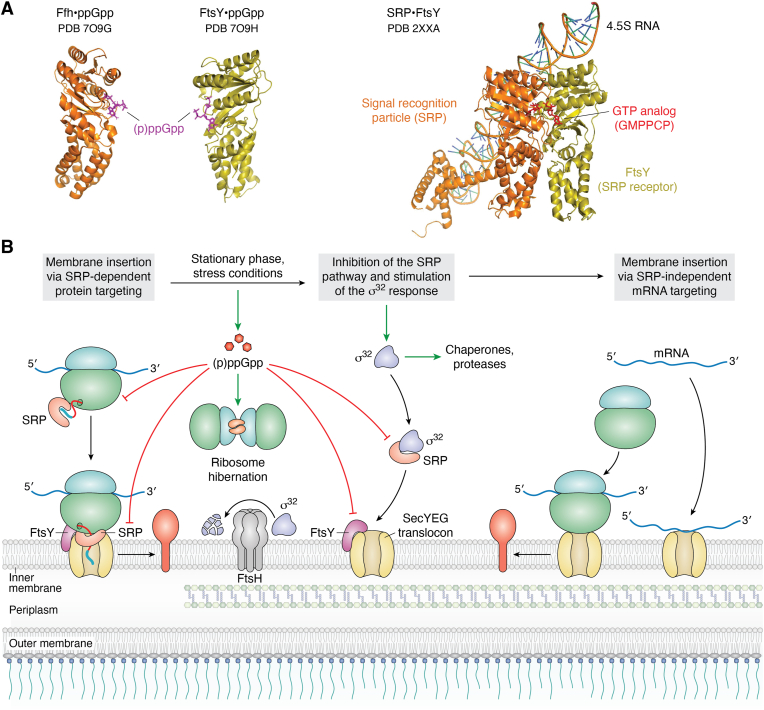
Figure 6.
SRP-dependent and SRP-independent membrane protein insertion in E. coli.A, structures of the ppGpp-bound Ffh (orange) and the pppGpp-bound SRP receptor FtsY (olive). (p)ppGpp (magenta) binding to Ffh and FtsY prevents the formation of the SRP–FtsY targeting complex (right panel), which is essential for both co- and posttranslational protein targeting by SRP. The targeting complex consists of the signal recognition particle (Ffh + 4.5S RNA) and its receptor FtsY and is shown in the presence of the GTP analogue GMPPCP (red). Structures were retrieved from the protein database (PDB) with the following IDs: 7O9G (Ffh in complex with ppGpp), 7O9H (FtsY in complex with pppGpp), 2XXA (Signal Recognition Particle [SRP] in complex with its receptor FtsY), and are depicted using Pymol. B, schematic representation of SRP-dependent and SRP-independent membrane protein insertion via mRNA targeting in E. coli. During stationary phase or when cells encounter stress conditions, the (p)ppGpp levels increase, which inhibits the SRP pathway, induces ribosome hibernation, and prevents the SRP-dependent membrane targeting of σ32. This reduces σ32 degradation by FtsH and stimulates the σ32-dependent production of chaperones and proteases. As a consequence of the inhibition of the SRP pathway, some stress-responsive membrane proteins, such as YohP, engage a translation-independent mRNA targeting pathway, which targets mRNAs directly to the SecYEG translocon or YidC, where they are translated by ribosomes and membrane inserted independently of the SRP pathway.