TABLE 1.
Optimization of reaction conditions. a
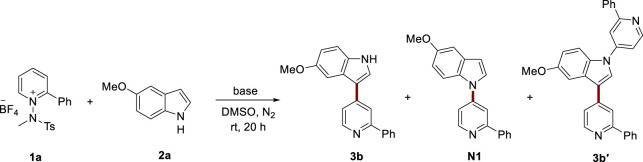
|
| Entry | 1a (equiv) | 2a (equiv) | Base (equiv) | Yield b (3b/N1/3b′) (%) |
|---|---|---|---|---|
| 1 | 1.5 | 1.0 | Cs2CO3 (2.0) | trace/trace/trace |
| 2 | 1.5 | 1.0 | NaOtBu (2.0) | 48/17/9 |
| 3 | 1.5 | 1.0 | DBU (2.0) | 38/trace/trace |
| 4 | 1.5 | 1.0 | NaH (2.0) | 51/13/12 |
| 5 | 1.5 | 1.0 | KOtBu (2.0) | 26/9/5 |
| 6 | 1.5 | 1.0 | LiOtBu (2.0) | 48/3/7 |
| 7 | 1.0 | 1.0 | LiOtBu (1.2) | 46/6/4 |
| 8 | 1.0 | 1.0 | LiOtBu (1.5) | 49/5/4 |
| 9 | 1.0 | 1.0 | LiOtBu (1.8) | 55/5/5 |
| 10 | 1.0 | 1.0 | LiOtBu (2.0) | 57/5/4 |
| 11 | 1.0 | 1.0 | LiOtBu (2.5) | 55/4/4 |
| 12 | 1.0 | 1.5 | LiOtBu (2.0) | 73/8/trace |
| 13 | 1.0 | 2.0 | LiOtBu (2.0) | 82/8/trace |
| 14 c | 1.0 | 2.0 | LiOtBu (2.0) | 18/2/trace |
| 15 d | 1.0 | 2.0 | LiOtBu (2.0) | trace/trace/trace |
| 16 | 1.0 | 2.0 | no base | trace/trace/trace |
Entry 13 was selected as an optimal condition.
Reaction conditions: 1a or 2a was used as a limiting reagent (0.1 mmol for 1.0 equiv) in DMSO (1.0 mL) under N2 atmosphere at rt for 20 h.
NMR, yields were measured with the caffeine as an internal standard.
DMF, was used instead of DMSO.
THF, was used instead of DMSO.
