Summary
Background
Novel oral poliovirus vaccine type 2 (nOPV2) was developed by modifying the Sabin strain to increase genetic stability and reduce risk of seeding new circulating vaccine-derived poliovirus type 2 outbreaks. Bivalent oral poliovirus vaccine (bOPV; containing Sabin types 1 and 3) is the vaccine of choice for type 1 and type 3 outbreak responses. We aimed to assess immunological interference between nOPV2 and bOPV when administered concomitantly.
Methods
We conducted an open-label, non-inferiority, randomised, controlled trial at two clinical trial sites in Dhaka, Bangladesh. Healthy infants aged 6 weeks were randomly assigned (1:1:1) using block randomisation, stratified by site, to receive nOPV2 only, nOPV2 plus bOPV, or bOPV only, at the ages of 6 weeks, 10 weeks, and 14 weeks. Eligibility criteria included singleton and full term (≥37 weeks’ gestation) birth and parents intending to remain in the study area for the duration of study follow-up activities. Poliovirus neutralising antibody titres were measured at the ages of 6 weeks, 10 weeks, 14 weeks, and 18 weeks. The primary outcome was cumulative immune response for all three poliovirus types at the age of 14 weeks (after two doses) and was assessed in the modified intention-to-treat population, which was restricted to participants with adequate blood specimens from all study visits. Safety was assessed in all participants who received at least one dose of study product. A non-inferiority margin of 10% was used to compare single and concomitant administration. This trial is registered with ClinicalTrials.gov, NCT04579510.
Findings
Between Feb 8 and Sept 26, 2021, 736 participants (244 in the nOPV2 only group, 246 in the nOPV2 plus bOPV group, and 246 in the bOPV only group) were enrolled and included in the modified intention-to-treat analysis. After two doses, 209 (86%; 95% CI 81–90) participants in the nOPV2 only group and 159 (65%; 58–70) participants in the nOPV2 plus bOPV group had a type 2 poliovirus immune response; 227 (92%; 88–95) participants in the nOPV2 plus bOPV group and 229 (93%; 89–96) participants in the bOPV only group had a type 1 response; and 216 (88%; 83–91) participants in the nOPV2 plus bOPV group and 212 (86%; 81–90) participants in the bOPV only group had a type 3 response. Co-administration was non-inferior to single administration for types 1 and 3, but not for type 2. There were 15 serious adverse events (including three deaths, one in each group, all attributable to sudden infant death syndrome); none were attributed to vaccination.
Interpretation
Co-administration of nOPV2 and bOPV interfered with immunogenicity for poliovirus type 2, but not for types 1 and 3. The blunted nOPV2 immunogenicity we observed would be a major drawback of using co-administration as a vaccination strategy.
Funding
The US Centers for Disease Control and Prevention.
Introduction
The trivalent oral poliovirus vaccine (tOPV; containing poliovirus types 1, 2, and 3) was adopted as the vaccine of choice for polio eradication.1 With the use of tOPV and other oral poliovirus vaccines (OPVs), wild poliovirus type 2 (WPV2) and WPV type 3 (WPV3) were certified as eradicated, with WPV2 last detected in 1999 and WPV3 in 2012; only WPV type 1 (WPV1) remains endemic, with circulation in reservoirs in Afghanistan and Pakistan and recent importations into other countries.2 However, as wild poliovirus elimination progressed, the emergence of circulating vaccine-derived poliovirus type 2 (cVDPV2) in areas without natural immunity and with poor childhood routine immunisation coverage with OPV— and relatively few supplementary immunisation activities with tOPV in WPV1 endemic countries—became a substantial challenge for the polio eradication programme.2
The Sabin strains in OPV are derived from wild polioviruses attenuated through serial passage in vivo and in vitro to reduce neurovirulence.3 OPV induces immune responses that closely parallel those of an infection with wild poliovirus. Poliovirus replicates primarily in the gastrointestinal tract and is excreted in pharyngeal secretions for several days and in faeces for several weeks, until development of antibodies in mucosae and blood interrupts replication.4,5 Antibodies in blood (humoral immunity) prevent paralysis, whereas the local intestinal immunity induced by OPV limits virus shedding, which interrupts poliovirus circulation, especially in settings with poor sanitation and hygiene where faecal–oral transmission predominates. OPV is also easy to administer by volunteers in mass campaigns. Vaccine virus might spread to close contacts, thus indirectly increasing vaccination coverage.3,6,7 The drawback of OPV use is that the vaccine virus strains can, in rare circumstances, lose their genetic attenuation during replication in under-vaccinated communities and regain the paralytic properties of wild poliovirus.8 Because of this possibility, eventual cessation of all Sabin OPV use will be necessary to achieve eradication.
As an initial step towards OPV cessation, in 2016, the Global Polio Eradication Initiative (GPEI) conducted a global switch from tOPV to bivalent OPV (bOPV; Sabin types 1 and 3).9 Monovalent OPV type 2 (mOPV2; Sabin type 2) was available upon release by the WHO Director-General to interrupt cVDPV2 outbreaks and stockpiled to control future cVDPV2 outbreaks. Before the switch, countries also began introducing at least one dose of inactivated poliovirus vaccine into routine immunisation programmes to mitigate the risk of cVDPV2 outbreaks. In the first year following cessation of tOPV use, cVDPV2 outbreaks that had emerged before or near to the time of cessation were seen in the Democratic Republic of the Congo, Nigeria, Pakistan, and Syria.10 The number of outbreaks then increased to an unexpected degree due to previous low underlying routine immunisation coverage, an increase in the population without type 2 intestinal immunity, and poor surveillance causing delayed detection of outbreaks. Low-coverage mOPV2 outbreak responses also seeded new emergences both inside response zones and in areas that had not recently used mOPV2 (possible importations or mishandling of vaccines).8,10 By 2020, 1616 paralytic cVDPV2 cases were reported after the switch by 34 countries. Between January, 2021, and December, 2022, 1529 paralytic cases caused by cVDPV (1339 by cVDPV2) were reported from 35 countries, compared with 36 paralytic cases caused by WPV1 from four countries (GPEI data as of April 18, 2023).
The need for more stable OPVs to control poliovirus outbreaks prompted the development of additional type 2 vaccine strains with modifications in the Sabin type 2 genome designed to increase genetic stability during replication and reduce reversion to neuropathogenicity.11,12 In November, 2020, a novel OPV type 2 (nOPV2) candidate was authorised by WHO under an Emergency Use Listing after phase 1 and 2 trials indicated that it was safe, immunogenic, and genetically more stable than mOPV2.13–16 Use of nOPV2 for cVDPV2 outbreak responses began in March, 2021 and, as of March, 2023, more than 580 million nOPV2 doses have been administered.17 Results from genomic surveillance have confirmed the genetic stability of the vaccine under field use conditions.18
Concurrent circulation of cVDPV2 and other poliovirus types is likely in some areas with low vaccination coverage. Recent examples include outbreaks in Somalia (2018), the Philippines and Malaysia (2019–20), Afghanistan and Pakistan (2019–21), Yemen (2021), Mozambique (2022), and the Democratic Republic of the Congo (2022).2,8 tOPV can and has been used in selected countries to control outbreaks with type co-circulation. As an alternative, concomitant administration of nOPV2 and bOPV could reduce the risk of seeding new vaccinederived polioviruses due to the increased genetic stability of nOPV2 compared with Sabin OPV2. The GPEI decided that during the initial use period the two vaccines (nOPV2 and bOPV) must be administered in separate rounds; however, co-administration could reduce the number of outbreak response rounds necessary to achieve high population immunity to all poliovirus types and, therefore, accelerate outbreak control with lower operational costs. To assess whether there is immunological interference between Sabin type 1 and type 3 and nOPV2 strains, we compared the humoral immune response to nOPV2 and bOPV administered together or separately among poliovirus vaccine-naive infants. We also assessed poliovirus excretion in stool specimens following the first vaccination as a measure of response to vaccine (ie, vaccine take).
Methods
Study design
We conducted an open-label, non-inferiority, randomised, controlled trial at two clinical trial sites in urban Dhaka, Bangladesh (Mirpur and Mohakhali). This study was reviewed and approved by the Institutional Review Board of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b). The protocol was shared with the US Centers for Disease Control and Prevention (CDC; Atlanta, GA, USA) but deferred to the icddr,b’s Institutional Review Board.
Participants
Study staff identified eligible infants through active surveillance of new births in the study communities surrounding the two sites. Eligibility criteria during enrolment included singleton and full term (≥37 weeks’ gestation) birth, age of 6 weeks (42–48 days), and parents intending to remain in the study area for the duration of study follow-up activities (ie, until the infant reached the age of 18 weeks). Additionally, infants were required to have a sibling younger than 10 years who was eligible to participate as a contact in the household transmission component of the trial (results to be reported separately). Exclusion criteria were evidence or suspicion of a medical condition that contraindicated blood collection or study vaccine administration and receipt of any poliovirus vaccine before enrolment. Written informed consent for participation was obtained from parents. Infants were withdrawn from the trial if blood could not be collected during the enrolment visit, if parents withdrew consent, if infants received poliovirus vaccines outside the trial, or if a medical condition was identified that contraindicated participation in the trial. Trial enrolment began in February, 2021, and follow-up activities concluded in December, 2021.
Randomisation and masking
Participants were randomly assigned (in a 1:1:1 allocation ratio) to one of three groups using a block randomisation scheme with varying block sizes of three, six, and nine, stratified by study site. The randomisation algorithm was generated by the US CDC using the blockrand package in R (version 4.0.3) and executed in the REDCap database (hosted by Vanderbilt University, Nashville, TN, USA) by study staff. Randomisation assignments were only revealed to study staff after completion of enrolment procedures. Laboratory staff were masked to study group assignments. The three groups were nOPV2 only, nOPV2 plus bOPV, and bOPV only; study vaccines were administered at the ages of 6 weeks, 10 weeks, and 14 weeks.
Procedures
Infants were enrolled at age 6 weeks and returned to the clinics at the ages of 10 weeks, 14 weeks, and 18 weeks. At each clinic visit, study staff collected information on clinical history, conducted physical examinations including weight and length measurements, collected one blood sample (1 mL) by venipuncture, administered study vaccines (except during the visit at age 18 weeks), and monitored infants for adverse events. Mean weight (precision 100 g) and length (precision 1 mm) measurements from two readings were used to determine whether participants had evidence of wasting (ie, reduced weight for length) or stunting (ie, reduced length for age) according to the child-growth standard curves from the WHO Multicentre Growth Reference Study.19 Wasting and stunting were defined as present if a participant’s measurements were more than 2 SD below the mean of the reference population. Blood samples were collected before administration of study vaccines. Participants also received all scheduled vaccines according to the Expanded Programme on Immunization (EPI) of the Bangladesh Ministry of Health and Family Welfare (except for poliovirus vaccines), including the pentavalent vaccine (diphtheria, pertussis, tetanus, hepatitis B, and Haemophilus influenzae type B) and the pneumococcal conjugate vaccine (Streptococcus pneumoniae). Stool samples were collected for assessment of viral shedding, household transmission of vaccine virus, and recombination (transmission and recombination results to be reported separately). Household stool collection took place at day 7 (only for the nOPV2 only group), and days 14 and 28 (all groups) after the first vaccination at age 6 weeks. Stool collection kits were delivered 1 day before the scheduled collection date. Mothers were instructed to collect and place the stool sample in a prelabelled container, store it in a cool place in the home, and notify fieldworkers immediately. Fieldworkers retrieved stool samples within 2 h of collection and transported them to the study clinic within 30 min of pick-up.
The nOPV2 and bOPV used in the trial were manufactured by PT Bio Farma (Bandung, Indonesia). nOPV2 is a live-attenuated type 2 poliovirus14 that was derived from a modified Sabin type 2 infectious cDNA clone and propagated in Vero cells. Each two-drop nOPV2 dose contained approximately 106·0 cell culture infective dose (CCID50) units of type 2 poliovirus. Each two-drop bOPV dose contained at least 105·8 CCID50 of Sabin type 1 and at least 105·8 CCID50 of Sabin type 3. All vaccine vials were single use and were administered with a supplied dropper. After completing study participation, infants received two doses of fractional inactivated poliovirus vaccine at the ages of 18 weeks and 26 weeks, and the nOPV2 only group and nOPV2 plus bOPV group received three doses of bOPV at 4-week intervals to comply with national EPI guidance. All study data were collected in REDCap.
Blood and stool samples were transported to the icddr,b laboratory by the end of each day (stored and transported at 2–8°C). Blood samples were centrifuged within 24 h of collection, and sera were aliquoted for testing (stored at −20°C) and storage (stored at −70°C). Stool samples were aliquoted for testing and storage and maintained at −20°C. Serum and stool specimens were sent to the US CDC for testing. Poliovirus neutralising antibody titres against all three poliovirus types were assessed using a polio microneutralisation assay20 with an upper limit of detection of 1:1448. The presence or absence of viral excretion in stool specimens was determined by real-time RT-PCR.14,21,22
Outcomes
The primary outcome was cumulative immune response measured at the age of 14 weeks, after receipt of two doses (this number of doses was chosen because GPEI recommends at least two vaccination rounds for outbreak response). Immune response was defined as seroconversion from seronegative (<1:8 titre) to seropositive (≥1:8 titre) or a 4-fold increase in titres among seropositive participants (adjusted for the exponential decay in maternal antibodies assuming a half-life of 28 days),23 between baseline and 4 weeks after each vaccination. In addition, to meet the definition of an immune response, seropositivity (ie, ≥1:8) had to be sustained up to age 18 weeks. Cumulative immune response was defined as an immune response at any point up to and including the time of assessment. Secondary outcomes were immune response after one and three doses, median reciprocal antibody titres, presence of faecal viral shedding after the first dose, household transmission of nOPV2, and assessment of recombination (transmission and recombination results to be reported separately because analyses are ongoing).
Vaccine safety was monitored throughout the course of the study. Adverse events were defined as any illness occurring in participants during the trial period, and serious adverse events were defined as death, a life-threatening event, hospitalisation or prolongation of existing hospitalisation, paralysis or severe disability, and anaphylaxis. Participants were monitored for 30 min post vaccination, and parents were asked about recent illness during clinic visits. Adverse events were also monitored throughout the course of the study, and parents were instructed to seek medical attention immediately and notify the study clinic if their infant became ill between study clinic visits. As an extra safety measure, a study physician solicited adverse events at one timepoint for each participant, 24–48 h after the first vaccination visit. The principal investigator (KZ) reviewed all adverse events reports, and all serious adverse events were reported within 24 h to icddr,b’s Institutional Review Board, the Data Safety Monitoring Board, and the CDC.
Statistical analysis
The sample size for this study was calculated to address the primary objectives. To assess the non-inferiority of nOPV2 administered concomitantly with bOPV compared with either vaccine given alone, we assumed that approximately 90% of participants would show a type 2 immune response after two doses of nOPV2,14 and approximately 85% would have a type 1 poliovirus immune response and at least 85% would have a type 3 response after two doses of bOPV.24,25 Accounting for 10% attrition, we calculated under the alternative hypothesis of non-inferiority that the enrolment target of 265 participants per group (795 total) would be sufficient to reject a non-inferiority margin of 10% in immune response proportions between groups, with a power of 90% and one-sided α value of 0·05.
Descriptive analyses of baseline characteristics and adverse events were also performed. Safety results are presented for all participants who received at least one dose of study product. Baseline poliovirus antibodies were presumed to represent maternal antibodies. We assessed non-inferiority by comparing the lower bound of the 90% two-sided CI for the proportion difference between groups to the noninferiority margin. The 95% Wilson (score) CIs were reported for seroconversion and proportion of participants with faecal shedding. The Kruskal-Wallis test was used to assess differences in measured reciprocal antibody titre distributions among responders between groups. The χ2 test was used to assess differences in the proportion of participants with viral shedding (defined as the presence of viral excretion in stool specimens). Multiple comparison correction was not applied to the analyses because a priori hypotheses were investigated at different outcome endpoints. Reverse cumulative distribution function curves were created to visualise the differences in reciprocal antibody titres among infants with an immune response.
The primary analytical approach was modified intention to treat, including participants who had adequate blood specimens for serology at baseline and at the ages of 10 weeks, 14 weeks, and 18 weeks. The secondary analytical approach was per protocol. Analyses were completed using R (version 4.0.3). This trial is registered with ClinicalTrials.gov, NCT04579510.
Role of the funding source
The funder of the study participated in the study design, data analysis, data interpretation, and writing of the report. The funder of the study had no role in data collection.
Results
Between Feb 8 and Sept 26, 2021, 934 parents were approached, and 795 infants were enrolled in the study (figure 1). Of these 795 infants, 736 (93%) were included in the modified intention-to-treat analysis: 244 in the nOPV2 only group, 246 in the nOPV2 plus bOPV group, and 246 in the bOPV only group. Baseline characteristics of participants are summarised in table 1.
Figure 1: Trial profile.
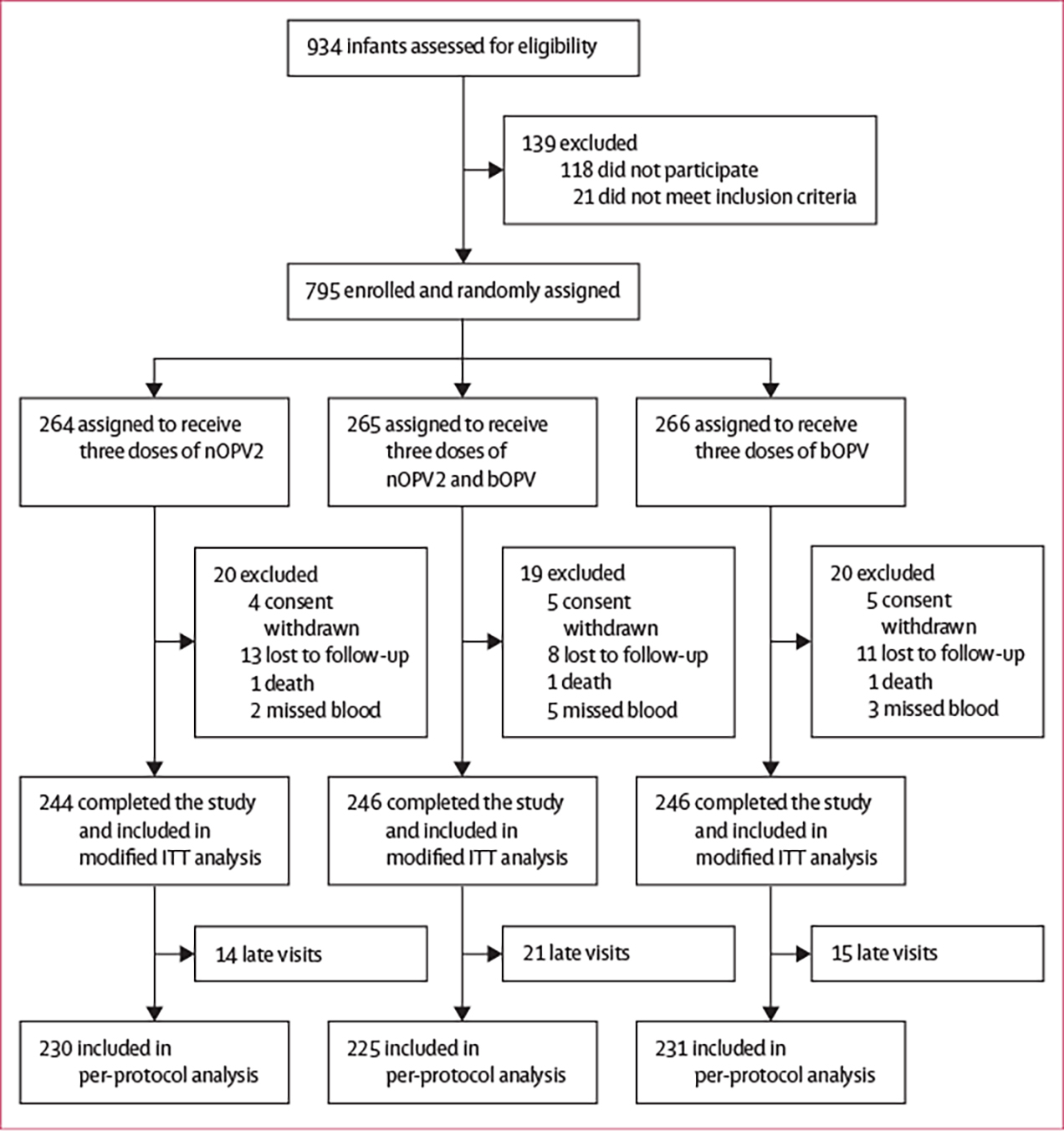
bOPV=bivalent oral poliovirus vaccine. ITT=intention-to-treat. nOPV2=novel oral poliovirus vaccine type 2.
Table 1:
Baseline characteristics of the modified intention-to-treat population
| nOPV2 only group (n=244) | nOPV2 plus bOPV group (n=246) | bOPV only group (n=246) | |
|---|---|---|---|
|
| |||
| Age, days | 44 (43–46) | 44 (43–46) | 45 (43–47) |
| Sex | |||
| Female | 110 (45%) | 114 (46%) | 131 (53%) |
| Male | 134 (55%) | 132 (54%) | 115 (47%) |
| Mother’s education | |||
| No formal school | 89 (36%) | 80 (33%) | 81 (33%) |
| Primary school | 73 (30%) | 74 (30%) | 69 (28%) |
| Middle school | 44 (18%) | 52 (21%) | 47 (19%) |
| High school | 32 (13%) | 34 (14%) | 43 (17%) |
| Graduate | 6 (2%) | 6 (2%) | 6 (2%) |
| Exclusive breastfeeding | 77 (32%) | 66 (27%) | 70 (28%) |
| Wasting present | 7 (3%) | 12 (5%) | 8 (3%) |
| Stunting present | 29 (12%) | 22 (9%) | 31 (13%) |
| Type 1 poliovirus | |||
| Seropositive | 92 (38%) | 111 (45%) | 116 (47%) |
| Reciprocal antibody titres | 23 (11–72) | 28 (11–72) | 18 (11–57) |
| Type 2 poliovirus | |||
| Seropositive | 134 (55%) | 144 (59%) | 137 (56%) |
| Reciprocal antibody titres | 23 (14–43) | 18 (11–45) | 23 (11–45) |
| Type 3 poliovirus | |||
| Seropositive | 69 (28%) | 72 (29%) | 78 (32%) |
| Reciprocal antibody titres | 18 (9–45) | 20 (11–48) | 18 (11–54) |
Data are median (IQR) or n (%). Baseline measurements for participants were obtained at 6 weeks of age and seropositive was defined as having an antibody titre of at least 1:8. bOPV=bivalent oral poliovirus vaccine. nOPV2=novel oral poliovirus vaccine type 2.
After two doses, a type 2 cumulative immune response was observed in 209 (86%; 95% CI 81–90) of 244 participants in the nOPV2 only group and 159 (65%; 58–70) of 246 participants in the nOPV2 plus bOPV group; after three doses, a type 2 cumulative immune response was observed in 225 (92%; 88–95) participants in the nOPV2 only group and 193 (78%; 73–83) participants in the nOPV2 plus bOPV group (table 2). Type 2 reciprocal antibody titre distributions among responders were also significantly lower in participants in the nOPV2 plus bOPV group than in those in the nOPV2 only group (appendix pp 2–3). At all timepoints, non-inferiority was rejected for a type 2 immune response of concomitantly administered nOPV2 and bOPV compared with nOPV2 given alone (figure 2). We report immunogenicity results from the per-protocol analysis in the appendix (pp 4–5); results did not differ.
Table 2:
Immune response for poliovirus types 1, 2, and 3 by study group in the modified intention-to-treat population
| nOPV2 only group (n=244) | nOPV2 plus bOPV group (n=246) | bOPV only group (n=246) | |
|---|---|---|---|
|
| |||
| Poliovirus type 1 | |||
| Immune response at the age of 10 weeks after one dose | 2 (1%; 0–3) | 191 (78%; 72–83) | 193 (78%; 73–83) |
| Cumulative immune response by the age of 14 weeks after two doses | 10 (4%; 2–7) | 227 (92%; 88–95) | 229 (93%; 89–96) |
| Cumulative immune response by the age of 18 weeks after three doses | 20 (8%; 5–12) | 240 (98%; 95–99) | 240 (98% [95–99) |
| Poliovirus type 2 | |||
| Immune response at the age of 10 weeks after one dose | 156 (64%; 58–70) | 72 (29%; 24–35) | 0 (0%; 0–2) |
| Cumulative immune response by the age of 14 weeks after two doses | 209 (86%; 81–90) | 159 (65%; 58–70) | 5 (2%; 1–5) |
| Cumulative immune response by the age of 18 weeks after three doses | 225 (92%; 88–95) | 193 (78%; 73–83) | 20 (8%; 5–12) |
| Poliovirus type 3 | |||
| Immune response at the age of 10 weeks after one dose | 3 (1%; 0–4) | 160 (65%; 59–71) | 148 (60%; 54–66) |
| Cumulative immune response by the age of 14 weeks after two doses | 6 (2%; 1–5) | 216 (88%; 83–91) | 212 (86%; 81–90) |
| Cumulative immune response by the age of 18 weeks after three doses | 8 (3%; 2–6) | 233 (95%; 91–97) | 231 (94%; 90–96) |
Data are the number of vaccine responders (% [95% CI]). bOPV=bivalent oral poliovirus vaccine. nOPV2=novel oral poliovirus vaccine type 2.
Figure 2: Non-inferiority assessment of immune response to poliovirus types 1, 2, and 3 in the modified intention-to-treat population.
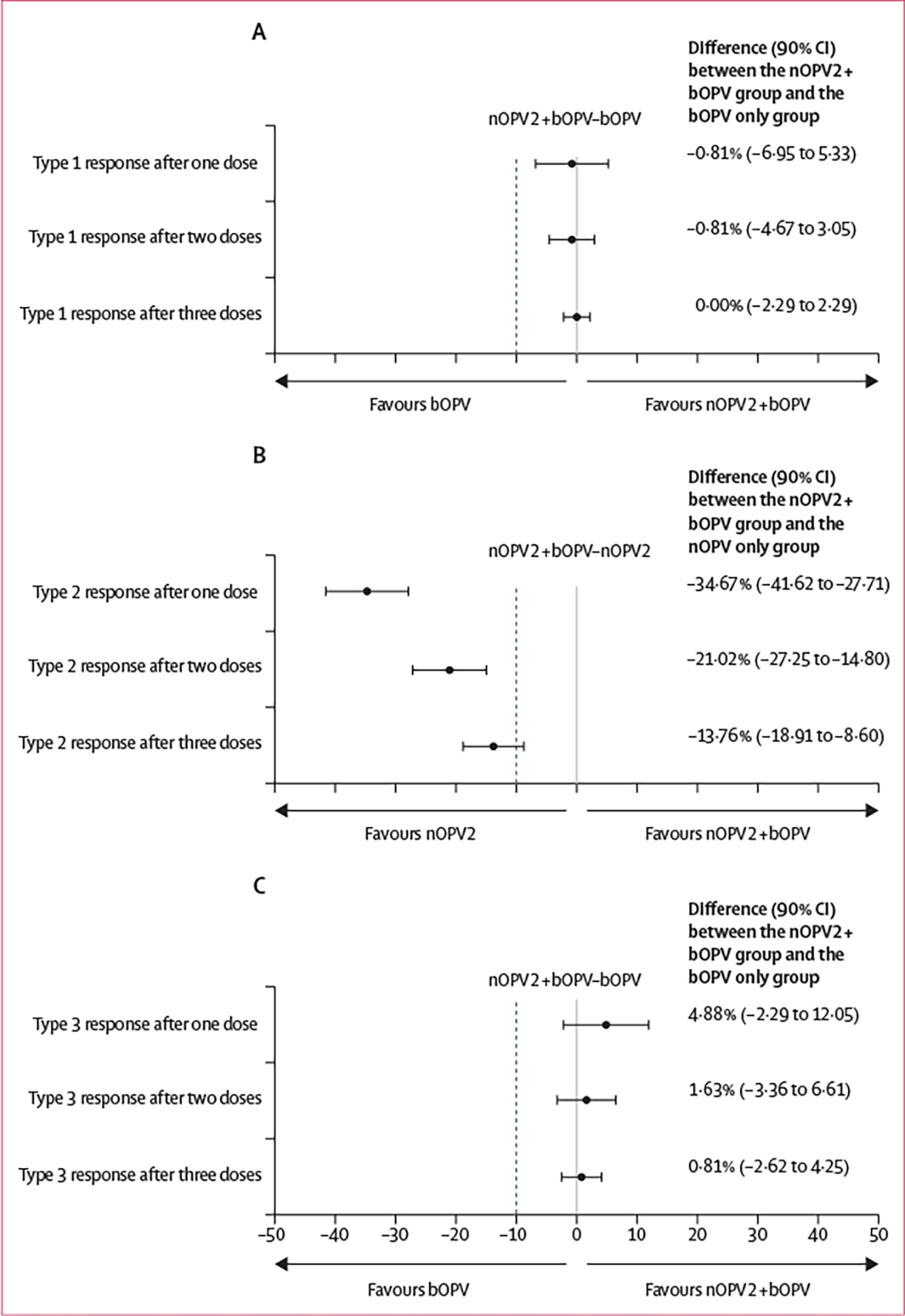
Differences in vaccine response are presented along with 90% CIs around the estimated difference. The dashed line represents the non-inferiority margin, defined as −10%. Non-inferiority is concluded if the lower bound of the 90% CI falls to the right of the non-inferiority margin. (A) Type 1, nOPV2, and bOPV (group B) in comparison with bOPV only (group C). (B) Type 2, nOPV2, and bOPV (group B) in comparison with nOPV2 only (group A). (C) Type 3, nOPV2, and bOPV (group B) in comparison with bOPV only (group C). nOPV2=novel oral poliovirus vaccine type 2. bOPV=bivalent oral poliovirus vaccine.
After two doses, a type 1 cumulative immune response was observed in 227 (92%; 95% CI 88–95) of 246 participants in the nOPV2 plus bOPV group and 229 (93%; 89–96) of 246 participants in the bOPV group; a type 3 response was observed in 216 (88%; 83–91) participants in the nOPV2 plus bOPV group and 212 (86%; 81–90) participants in the bOPV only group (table 2). After three doses, 240 (98%) participants in the nOPV2 plus bOPV group and 240 (98%) participants in the bOPV only group had a type 1 response and 233 (95%) participants in the nOPV2 plus bOPV group and 231 (94%) participants in the bOPV only group had a type 3 response (table 2). Median type 1 reciprocal antibody titres were 1448 or higher for both groups at all timepoints. For type 3, titre distributions after two and three doses were significantly lower in the nOPV2 plus bOPV group than in the bOPV only group (appendix pp 2–3). The difference in type 1 and 3 immune responses to concomitantly administered nOPV2 and bOPV were non-inferior compared with bOPV given alone at all timepoints (figure 2).
In the nOPV2 only group, after the first nOPV2 dose, type 2 poliovirus shedding was seen in 168 (69%; 95% CI 63–75) of 244 infants at 7 days, 161 (67%; 61–72) of 242 infants at 14 days, and 116 (48%; 41–54) of 244 infants by 28 days (table 3). Of the 240 participants in the nOPV2 only group with all three stool samples collected, 168 (70%) were shedding type 2 poliovirus at any timepoint. Of infants in the nOPV2 plus bOPV group, 87 (36%; 95% CI 30–42) of 245 were shedding type 2 poliovirus at 14 days (p<0·0001 compared with the nOPV2 only group) and 73 (30%; 24–35) of 246 at 28 days (p<0·0001 compared with the nOPV2 only group). There were no significant differences in type 1 and type 3 poliovirus shedding between the nOPV2 plus bOPV group and bOPV only group at either 14 days or 28 days after first vaccination (table 3). The proportion of infants excreting poliovirus in their stool either 14 days or 28 days after the first dose of nOPV2 (restricted to infants with results available for both stool specimens) was similar to the proportion that developed an immune response. Among infants with type 2 poliovirus excretion at either 14 days or 28 days, 153 (94%) of 162 in the nOPV2 only group and 71 (72%) of 98 in the nOPV2 plus bOPV group had a type 2 immune response at the age of 10 weeks.
Table 3:
Faecal shedding of poliovirus 7, 14, and 28 days after first vaccination by study group in the modified intention-to-treat population
| nOPV2 only group | nOPV2 plus bOPV group | bOPV only group | χ2 test | |
|---|---|---|---|---|
|
| ||||
| Poliovirus type 1 | ||||
| Day 7 | 2/244 (1%; 0–2) | .. | .. | NA |
| Day 14 | 5/242 (2%; 0–4) | 125/245 (51%; 45–57) | 141/245 (58%; 51–64) | nOPV2 plus bOPV vs bOPV only: p=0.17 |
| Day 28 | 3/244 (1%; 0–3) | 64/246 (26%; 21–31) | 77/246 (31%; 26–37) | nOPV2 plus bOPV vs bOPV only: p=0.23 |
| Poliovirus type 2 | ||||
| Day 7 | 168/244 (69%; 63–75) | .. | .. | NA |
| Day 14 | 161/242 (67%; 61–72) | 87/245 (36%; 30–42) | 3/245 (1%; 0–3) | nOPV2 only vs nOPV2 plus bOPV: p<0.0001 |
| Day 28 | 116/244 (48%; 41–54) | 73/246 (30%; 24–35) | 2/246 (1%; 0–2) | nOPV2 only vs nOPV2 plus bOPV: p<0.0001 |
| Poliovirus type 3 | ||||
| Day 7 | 7/244 (3%; 1–5) | .. | .. | NA |
| Day 14 | 8/242 (3%; 1–6) | 170/245 (69%; 64–75) | 151/245 (62%; 56–68) | nOPV2 plus bOPV vs bOPV only: p=0.087 |
| Day 28 | 8/244 (3%; 1–6) | 136/246 (55%; 49–61) | 131/246 (53%; 47–59) | nOPV2 plus bOPV vs bOPV only: p=0.72 |
Data are n/N (%; 95% CI). p values are reported for comparisons of interest (ie, they include comparisons between study vaccines without a specific type). bOPV=bivalent oral poliovirus vaccine. nOPV2=novel oral poliovirus vaccine type 2. NA=not applicable.
In total, 64 adverse events were reported in 61 participants (of 795 participants who received at least one dose of study product; table 4). 15 (23%) of the 64 adverse events were classified as serious, including three deaths attributed to sudden infant death syndrome (one in each group). Unsolicited adverse events were reported in 17 (6%) of 264 participants in the nOPV2 only group, 16 (6%) of 265 participants in the nOPV2 plus bOPV group, and 16 (6%) of 266 participants in the bOPV only group (table 4). 16 solicited adverse events, ten of which were fever, were reported in 13 participants during the clinician’s telephone call 24–48 h after the first vaccination (table 4). All adverse events were deemed to be unrelated to the study vaccines.
Table 4:
Adverse events reported in participants during the study, by study group
| nOPV2 only group (n=264) | nOPV2 plus bOPV group (n=265) | bOPV only group (n=266) | |
|---|---|---|---|
|
| |||
| Any adverse event | 24 (9%) | 17 (6%) | 20 (8%) |
| Any serious adverse event | 5 (2%) | 4 (2%) | 6 (2%) |
| Unsolicited adverse events | |||
| Any | 17 (6%) | 16 (6%) | 16 (6%) |
| Acute respiratory infections | 6 (2%) | 8 (3%) | 7 (3%) |
| Acute diarrhoea and gastroenteritis | 4 (2%) | 4 (2%) | 3 (1%) |
| Pneumonia | 5 (2%) | 3 (1%) | 2 (1%) |
| Death, SIDS | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Cardiomyopathy | 0 | 0 | 1 (<1%) |
| Dengue | 0 | 0 | 1 (<1%) |
| Fever | 0 | 0 | 1 (<1%) |
| Malnutrition | 0 | 1 (<1%) | 0 |
| Oral thrush | 1 (<1%) | 0 | 0 |
| Solicited adverse events | |||
| Any | 7 (3%) | 1 (<1%) | 5 (2%) |
| Fever | 5 (2%) | 1 (<1%) | 4 (2%) |
| Abnormal crying | 0 | 1 (<1%) | 1 (<1%) |
| Vomiting | 1 (<1%) | 0 | 1 (<1%) |
| Poor feeding | 1 (<1%) | 0 | 0 |
| Other (diarrhoea) | 1 (<1%) | 0 | 0 |
| Irritability | 0 | 0 | 0 |
| Drowsiness | 0 | 0 | 0 |
Data are n (%). bOPV=bivalent oral poliovirus vaccine. nOPV2=novel oral poliovirus vaccine type 2. SIDS=sudden infant death syndrome.
Discussion
Our findings indicate that concomitant administration of nOPV2 and bOPV reduces nOPV2 immunogenicity but does not significantly affect bOPV immunogenicity. After two doses of co-administered nOPV2 and bOPV, 65% of infants had a type 2 immune response compared with 86% when nOPV2 was administered alone; type 2 antibody titres among responders were also consistently lower in the co-administration group. Co-administration resulted in reduced stool excretion of poliovirus type 2, but not of types 1 and 3, suggesting that the Sabin type 1 or 3 strains interfere with intestinal replication of the nOPV2 strain and thereby blunt the type 2 systemic humoral immune response (and probably the mucosal immune response). Even after three doses, type 2 immunogenicity in the co-administration group was significantly lower than that observed with nOPV2 alone. These immune response rates in nOPV2 recipients were also lower than those reported in Bangladesh in recipients of mOPV226 or tOPV from other studies.27
One possible reason for our findings is that genetic modifications present in nOPV2 reduce its fitness for intestinal replication, particularly relative to Sabin types 1 and 3 when they are administered together. Interference among Sabin strains was observed in the early 1960s when mOPVs were replaced by a tOPV.3 In a tOPV preparation with the same dose of all three Sabin types, Sabin type 2 predominated over types 1 and 3, resulting in lower intestinal excretion and antibody production of types 1 and 3.4 The lower immunogenicity of tOPV for types 1 and 3 was compensated through provision of several doses. Ultimately, changes were made in the formulation to an exponential dose ratio of 10:1:3 for types 1, 2, and 3.4,28 If nOPV2 and bOPV are given together, extra doses of nOPV2 would be needed to overcome the type 2 immune response interference mediated by types 1 and 3 to reach a high immune response rate. Monovalent nOPV1 and nOPV3 candidates are currently being evaluated in clinical trials (NCT04529538). A potential trivalent nOPV formulation might be able to compensate for the interference by optimising the titre ratios during formation of the three novel strains.
We observed lower immunogenicity for nOPV2 than that found in a study in Panama and similar results to a study conducted in Matlab, Bangladesh.13,29 The Matlab study reported a lower seroconversion rate after the first dose administered at birth (46%) than we reported (64%), consistent with the lower immunogenicity of doses given at birth, and similar seroconversion (90%, compared with 86% in our study) after two doses.29 In the Panama study, infants received three doses of bOPV and one dose of inactivated poliovirus vaccine before nOPV2, and the seroconversion rate after two nOPV2 doses was 98%.13 The seroprotection rate after one dose of nOPV2 was non-inferior to mOPV2 historical controls.13 Differences in results between studies might be related to differences in study design or differences in the presence of factors that interfere with OPV immunogenicity, including higher prevalence of diarrhoeal diseases and enterovirus infections and higher concentrations of maternal antibodies.3,27,30
However, when we compare our nOPV2 results to previous trials at the same sites in Bangladesh and following the same vaccination schedule, we observe higher type 2 immune response rates for mOPV2 and tOPV than we observed for nOPV2. For mOPV2, the immune response rate was 91% after one dose and the cumulative immune response was 97% after two doses;26 for tOPV it was 93% after two doses and 96% after three doses.27 The lower immunogenicity of nOPV2 in the current study versus Sabin OPV2 in previous trials might be at least partly explained by secondary OPV2 exposure from tOPV use in essential (routine) immunisation during the earlier trials. However, if nOPV2 is less immunogenic than mOPV2 in poliovirus vaccine-naive populations, as suggested by our findings, three doses might be required to protect at least 90% of immunologically naive infants, especially if risk factors for vaccine interference are prevalent, including recent or concomitant bOPV administration. Based on this finding, and because some children are missed during campaigns, we suggest further investigation as to whether two outbreak response rounds with nOPV2 is adequate to control cVDPV2 outbreaks, especially in populations with several years of OPV2-naive birth cohorts.
Our study has several limitations. Our estimates of type 1 and type 3 immune responses might have been affected in part by background exposure from bOPV use in the community in essential immunisation programmes, as shown in the nOPV2 only group, with type 1 and 3 immune response detection increasing by age. Since the trial was conducted in poliovirus vaccinenaive infants after OPV2 withdrawal, our estimates of type 2 responses would not be affected by broad secondary exposure to OPV2, but there evidently was some nOPV2 community transmission in the study area shown by a low level of type 2 immune responses in the bOPV only group. Another study limitation is that microneutralisation assays cannot distinguish between maternal antibodies and vaccine response antibodies, which, even with antibody decay adjustment, might have led to misclassification of immune response in either direction.
In conclusion, concomitant administration of nOPV2 with bOPV adversely affected nOPV2 immunogenicity, but not bOPV immunogenicity. On the basis of our findings, the operational benefits of co-administration to reduce the number of vaccination rounds necessary to achieve immunity to all types would need to be weighed against the reduced type 2 immunogenicity we observed. A co-administration strategy might be more attractive in scenarios with co-circulation of types when there is a need to achieve high population immunity to all types rapidly; however, this strategy should consider the possibility of planning an initial response of at least three rounds. Testing to assess recombination among viral strains and the concomitant risk of reversion when nOPV2 and bOPV are administered together is ongoing and will be presented separately. Our findings can inform outbreak response strategy decisions and considerations for when a trivalent nOPV product is being formulated. In addition, if nOPV2 is considered for introduction into essential immunisation in any location, this study can provide, to our knowledge, the first evidence of the decreased immune response when nOPV2 and bOPV are co-administered.
Supplementary Material
Research in context.
Evidence before this study
The Global Polio Eradication Initiative has supported the development of type 2 poliovirus vaccine strains with genetic modifications designed to reduce their ability to revert to neurovirulent variants compared with monovalent oral poliovirus vaccine type 2 (mOPV2). Phase 1 and 2 trials of novel oral poliovirus vaccine type 2 (nOPV2) candidates showed that the vaccines were safe and well tolerated, with similar immunogenicity to mOPV2. In November, 2020, an nOPV2 vaccine candidate was approved by WHO under an Emergency Use Listing. Field use of nOPV2 began in March, 2021 and, as of March, 2023, more than 580 million doses have been administered in campaigns in response to circulating vaccinederived poliovirus type 2 (cVDPV2) outbreaks. We searched PubMed to identify nOPV2 publications. We identified English language publications published before Aug 1, 2022, using the terms “nOPV2”, “novel OPV2”, “novel oral poliovirus vaccine”, and “novel type 2”. We selected all preclinical and clinical trials that included an nOPV2 vaccine candidate.
Added value of this study
Populations with low immunity are at risk of concurrent outbreaks of cVDPV2 and another poliovirus type. As a response strategy, concomitant administration of nOPV2 and bivalent oral poliovirus vaccine (bOPV) could raise population immunity to all three poliovirus types, reduce operational costs, and reduce the risk of seeding new cVDPV2 emergences compared with Sabin mOPV2 or trivalent oral poliovirus vaccine. To our knowledge, this open-label, non-inferiority, randomised, controlled trial is the first study to investigate the immunogenicity of co-administered nOPV2 and bOPV. We showed that, among poliovirus vaccine-naive infants, concomitant administration of nOPV2 and bOPV significantly reduces nOPV2 type 2 immunogenicity but does not significantly interfere with bOPV immunogenicity for types 1 and 3. Co-administration was also associated with lower rates of type 2 poliovirus shedding after the first nOPV2 vaccination, suggesting that bOPV interferes with intestinal replication of nOPV2.
Implications of all the available evidence
The available evidence supports the use of nOPV2 to respond to cVDPV2 outbreaks and reduce the risk of seeding new vaccinederived poliovirus emergences. Results from this study suggest that the operational advantages of nOPV2 and bOPV co-administration to control outbreaks with co-circulation of poliovirus types would need to be weighed against the reduced type 2 immunogenicity we observed. To our knowledge, our data are the only direct information available to inform decisions around concomitant use of nOPV2 and bOPV.
Acknowledgments
The study was funded by the Global Immunization Division of the US Centers for Disease Control and Prevention (CDC). The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the CDC. icddr,b acknowledges with gratitude the commitment of the CDC to its research efforts. icddr,b is also grateful to the Governments of Bangladesh, Canada, Sweden, and the UK for providing core and unrestricted support. We thank the study staff at the Mirpur and Mohakhali sites in Dhaka, Bangladesh, Warda Haque, and Mahmuda Khatun (laboratory support), and Sushil Dasgupta (data management support) at iccdr,b. We thank members of the Population Immunity Laboratory (William Hendley, Basit Jafri, Kathryn Jones, and Sharla McDonald) and Vaccine Development Laboratory (Ray Campagnoli, Larin McDuffie, Jing Shaw, Annelet Vincent, and Ling Wei) at the Polio and Picornavirus Laboratory Branch and Marla Petway and the Specimen Triage and Tracking Team for processing and testing the samples at the CDC. We also thank all parents, infants, and children who participated in this study. We thank Bio Farma for the donation of study vaccines.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Amanda L Wilkinson, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Khalequ Zaman, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Masuma Hoque, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Concepcion F Estivariz, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Cara C Burns, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Jennifer L Konopka-Anstadt, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Bernardo A Mainou, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Stephanie D Kovacs, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Qian An, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Jacquelyn S Lickness, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mohammad Yunus, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Cynthia J Snider, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Yiting Zhang, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Elizabeth Coffee, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Talha Abid, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Steven G F Wassilak, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Mark A Pallansch, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
M Steven Oberste, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
John F Vertefeuille, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Abhijeet Anand, US Centers for Disease Control and Prevention, Atlanta, GA, USA.
Data sharing
Aggregated data from tables 2–4 and the appendix (p 2) will be added to the ClinicalTrials.gov registration with publication. In accordance with the protocol, icddr,b investigators will have access to participant data with identifiers. External investigators will have access to de-identified participant data. De-identified data can be shared with national and international vaccine manufacturers and regulatory authorities upon request to the corresponding author.
References
- 1.Dowdle WR, Cochi SL. Global eradication of poliovirus: history and rationale. In: Semler BL, Wimmer E, eds. Molecular biology of picornaviruses. Washington, DC: ASM Press, 2002: 473–80. [Google Scholar]
- 2.Rachlin A, Patel JC, Burns CC, et al. Progress toward polio eradication—worldwide, January 2020–April 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 650–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutter R, Kew O, Cochi S, Aylward RB. Poliovirus vaccine—live. In: Plotkin S, Orenstein WA, Offit PA, eds. Vaccines, 6th edn. Philadelphia, PA: Elsevier Saunders, 2012. [Google Scholar]
- 4.Patriarca P, Linkins R, Sutter R, Orenstein W. Optimal schedule for the administration of oral poliovirus vaccine. In Kurstak E, ed. Measles and poliomyelitis, Austria: Springer-Verlag, 1993: 303–13. [Google Scholar]
- 5.Ogra PL, Fishaut M, Gallagher MR. Viral vaccination via the mucosal routes. Rev Infect Dis 1980; 2: 352–69. [DOI] [PubMed] [Google Scholar]
- 6.Estívariz CF, Pallansch MA, Anand A, et al. Poliovirus vaccination options for achieving eradication and securing the endgame. Curr Opin Virol 2013; 3: 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benyesh-Melnick M, Melnick JL, Rawls WE, et al. Studies of the immunogenicity, communicability and genetic stability of oral poliovaccine administered during the winter. Am J Epidemiol 1967; 86: 112–36. [DOI] [PubMed] [Google Scholar]
- 8.Alleman MM, Jorba J, Henderson E, et al. Update on vaccinederived poliovirus outbreaks—worldwide, January 2020–June 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1691–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampton LM, Farrell M, Ramirez-Gonzalez A, et al. Cessation of trivalent oral poliovirus vaccine and introduction of inactivated poliovirus vaccine—worldwide, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 934–38. [DOI] [PubMed] [Google Scholar]
- 10.Macklin GR, O’Reilly KM, Grassly NC, et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science 2020; 368: 401–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeh MT, Bujaki E, Dolan PT, et al. Engineering the live-attenuated polio vaccine to prevent reversion to virulence. Cell Host Microbe 2020; 27: 736–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konopka-Anstadt JL, Campagnoli R, Vincent A, et al. Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization. NPJ Vaccines 2020; 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sáez-Llorens X, Bandyopadhyay AS, Gast C, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials. Lancet 2021; 397: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Damme P, De Coster I, Bandyopadhyay AS, et al. The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a doubleblind, single-centre phase 1 study. Lancet 2019; 394: 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahid R, Mercer L, Gast C, et al. Evaluating stability of attenuated Sabin and two novel type 2 oral poliovirus vaccines in children. NPJ Vaccines 2022; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Coster I, Leroux-Roels I, Bandyopadhyay AS, et al. Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials. Lancet 2021; 397: 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandyopadhyay A Why is polio making a “comeback” and what can we do about it? 2023. https://speakingofmedicine.plos.org/2023/03/17/why-is-polio-making-a-comeback-and-what-can-we-do-about-it/ (accessed April 21, 2023). [Google Scholar]
- 18.Martin J, Burns CC, Jorba J, et al. Genetic characterization of novel oral polio vaccine type 2 viruses during initial use phase under emergency use listing—worldwide, March–October 2021. MMWR Morb Mortal Wkly Rep 2022; 71: 786–90. [DOI] [PubMed] [Google Scholar]
- 19.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization, 2006. [Google Scholar]
- 20.Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016; 1387: 145–76. [DOI] [PubMed] [Google Scholar]
- 21.Gerloff N, Sun H, Mandelbaum M, et al. Diagnostic assay development for poliovirus eradication. J Clin Microbiol 2018; 56: e01624–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gast C, Bandyopadhyay AS, Sáez-Llorens X, et al. Fecal shedding of 2 novel live attenuated oral poliovirus type 2 vaccine candidates by healthy infants administered bivalent oral poliovirus vaccine/inactivated poliovirus vaccine: 2 randomized clinical trials. J Infect Dis 2022; 226: 852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen-Abbo A, Culley BS, Reed GW, et al. Seroresponse to trivalent oral poliovirus vaccine as a function of dosage interval. Pediatr Infect Dis J 1995; 14: 100–06. [DOI] [PubMed] [Google Scholar]
- 24.Anand A, Zaman K, Estívariz CF, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: a randomized controlled trial. Vaccine 2015; 33: 6816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet 2010; 376: 1682–88. [DOI] [PubMed] [Google Scholar]
- 26.Zaman K, Estívariz CF, Morales M, et al. Immunogenicity of type 2 monovalent oral and inactivated poliovirus vaccines for type 2 poliovirus outbreak response: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18: 657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estívariz CF, Anand A, Gary HE Jr, et al. Immunogenicity of three doses of bivalent, trivalent, or type 1 monovalent oral poliovirus vaccines with a 2 week interval between doses in Bangladesh: an open-label, non-inferiority, randomised, controlled trial. Lancet Infect Dis 2015; 15: 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patriarca PA, Laender F, Palmeira G, et al. Randomised trial of alternative formulations of oral poliovaccine in Brazil. Lancet 1988; 1: 429–33. [DOI] [PubMed] [Google Scholar]
- 29.Zaman K, Bandyopadhyay AS, Hoque M, et al. Evaluation of the safety, immunogenicity, and faecal shedding of novel oral polio vaccine type 2 in healthy newborn infants in Bangladesh: a randomised, controlled, phase 2 clinical trial. Lancet 2023; 14: 131–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardemil CV, Estivariz C, Shrestha L, et al. The effect of diarrheal disease on bivalent oral polio vaccine (bOPV) immune response in infants in Nepal. Vaccine 2016; 34: 2519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Aggregated data from tables 2–4 and the appendix (p 2) will be added to the ClinicalTrials.gov registration with publication. In accordance with the protocol, icddr,b investigators will have access to participant data with identifiers. External investigators will have access to de-identified participant data. De-identified data can be shared with national and international vaccine manufacturers and regulatory authorities upon request to the corresponding author.


