Figure 1.
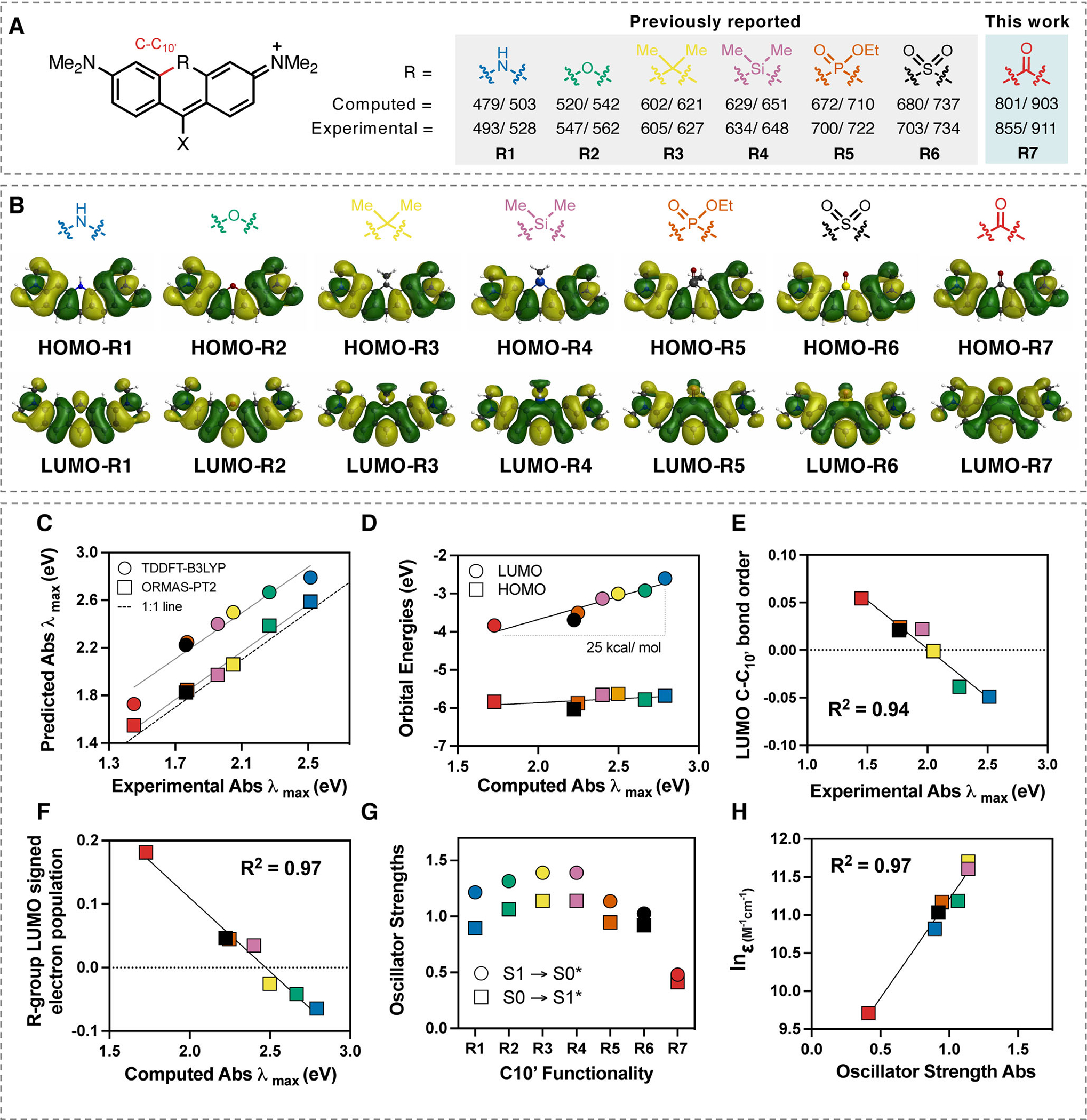
(A) Computational and experimental absorbance/emission maxima data for substituents (1–7) at R-position on a dimethylamino-xanthene-derived scaffold (see Table S1 for X-group substituent). (B) Comparisons between the HOMOs and LUMOs of R1-R7. (C) Correlation between experimental and predicted absorbance using TDDFT-B3LYP-PCM(H2O) and ORMAS-PT2-PCM(H2O). (D) Correlation between the computed absorbance (TDDFT-B3LYP-PCM(H2O)) and HOMO/LUMO orbital energies. (E) Correlation () between experimental absorbance and C–C10’ bond order in the LUMO. (F) Correlation () between signed R-group Mulliken population in LUMO and computed absorbance. (G) Comparison between computed absorbance and emission oscillator strengths (TDDFT-B3LYP-PCM(H2O)) for R1-R7. H) Comparison between computed absorbance oscillator strengths (TDDFT-B3LYP-PCM(H2O)) for R1-R7 and experimental extinction coefficient values for structurally related compounds (see Supporting Information for further details).
