Abstract
Purpose
Due to the spread of antimicrobial-resistant bacteria and poor penetration of many antimicrobial drugs across the blood–brain barrier following intravenous administration, treatment of central nervous system (CNS) infections is challenging, especially infections caused by carbapenem-resistant organisms (CRO). Intraventricular (IVT) infusion of antimicrobial drugs could be a choice. This report aims to describe a patient with CNS infection caused by carbapenem-resistant Acinetobacter baumannii (CRAB) which was successfully treated with IVT combined with intravenous (IV) colistin sulfate.
Methods
A case of CNS infection caused by CRAB after a craniocerebral injury was presented. The patient was treated with IVT together with IV colistin sulfate. Moreover, literature on the regimens and safety of colistin sulfate were also reviewed and summarized.
Results
Intraventricular (50,000 U, qd/100,000 U, qd) combined with IV (500,000 U, q12h/500,000 U, q8h) colistin sulfate was given to the patient, and the CNS infection was successfully controlled. The patient was finally transferred back to a local hospital for rehabilitation treatment. No nephrotoxicity or neurotoxicity was observed during the therapy.
Conclusion
IV combined with IVT colistin sulfate is effective in the treatment of CNS infections caused by CRAB. IVT concomitant IV colistin sulfate might be a therapeutic option worth considering in the treatment of CNS infections caused by CRO.
Keywords: central nervous system infection, Acinetobacter baumannii, intraventricular administration, colistin sulfate, colistin, polymyxin
Introduction
Central nervous system (CNS) infection is one of the common complications after neurosurgery. Published studies have reported the incidence of postoperative CNS infections after neurosurgical procedures to be 4.6%–25%.1 With the rapid spread of carbapenem-resistant organisms (CRO), CNS infections caused by these organisms are also increasing. However, CNS infections caused by CRO are challenging to treat due to the limited available antimicrobial options. Colistin-based combination therapy has become the last-line treatment of CNS infections caused by CRO.2 Currently, only three forms of polymyxins are clinically available in China, ie, colistimethate sodium (CMS), polymyxin B sulfate, and colistin sulfate. Of which, colistin sulfate was developed independently in China, by the Institute of Antibiotics, Chinese Academy of Medical Sciences in 1966. In 2011, the Shanghai Huashan Antibiotic Research Institute of Fudan University applied to the Shanghai Food and Drug Administration to resume production. After process improvements were carried out for the drug, colistin sulfate was re-introduced to the market in 2018. Numerous studies on polymyxins have been published, especially on CMS. Although colistin sulfate and CMS are both forms of polymyxin E, these two drugs should be distinguished. CMS is the form of inactive prodrug which needs to be converted into the active moiety (colistin) to exert its bactericidal effect. While colistin sulfate is an active form with bactericidal activity that does not require conversion. A study has shown that when given at the same dose, the nephrotoxicity of colistin sulfate is lower than that of CMS.3 Colistin sulfate has similar pharmacokinetic characteristics to that of polymyxin B sulfate.4 At present, the clinical efficacy and safety of polymyxin B have been widely reported, but studies on those of colistin sulfate are still limited. In this study, we report a case of CNS infection caused by carbapenem-resistant Acinetobacter baumannii (CRAB) after a craniocerebral injury. Based on the results of the antimicrobial susceptibility test and considering the penetration of antimicrobial agents into the CNS, the patient was treated with colistin sulfate (colistin sulfate for injection, Shanghai SPH New Asia Pharmaceutical Co. Ltd., Shanghai, China) intravenously and intraventricularly.
Case Report
A 55-year-old male patient with a body mass index (BMI) of 23.66 kg/m2 was admitted to our hospital on 10 January 2023. Twenty days ago, he fell from a height resulting in a severely closed craniocerebral injury. The patient underwent intracerebral hematoma evacuation and decompressive craniectomy in our hospital. Neurological nutrition, postoperative prophylaxis for infections and epilepsy, together with symptomatic treatment were given to the patient. He was subsequently discharged to a rehabilitation hospital for functional recovery after his condition stabilized. However, the patient presented with a decreased level of consciousness during the rehabilitation process. The pressure in the area of the right cranial craniotomy was high with significant protrusion. The patient was re-admitted to our hospital.
At admission, the patient was in a comatose state and unable to cooperate with the physical examination. Bilateral pupils were unequal, with left pupil of 2.5 mm and right pupil of 3.0 mm. The patient was slow in response to light reflex. The tracheotomy was unobstructed, with rough breathing sounds heard in the lungs. The patient’s neck had a strong grip with three fingers, while the muscle strength of all limbs was at level 1 and the muscle tension was high. Physiological reflexes were presented, and pathological signs were detected in the lower limbs. The clinical features of the patient at admission described in this report are listed in Table 1. The patient was clinically diagnosed with hydrocephalus following severe closed traumatic brain injury.
Table 1.
Clinical Characteristics of the Patient at Admission
| Parameters | Values |
|---|---|
| Temperature (T, °C) | 38 |
| Pulse rate (P, min−1) | 98 |
| Respiratory rate (R, min−1) | 20 |
| WBC (×109/L) | 11.87 |
| Neutrophils (percent, N%) | 93.6 |
| RBC (×1012/L) | 2.79 |
| GLU (mmol/L) | 9.97 |
| ALT (U/L) | 165 |
| AST (U/L) | 122 |
| ALB (g/L) | 29.8 |
| TBil (μmol/L) | 16.8 |
| DBil (μmol/L) | 11.1 |
| Creatinine (μmol/L) | 50 |
| Uric acid (μmol/L) | 142 |
| eGFR (mL/min) | 116.8 |
Abbreviations: WBC, white blood cell count; RBC, red blood cell count; GLU, glucose; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; TBil, total bilirubin; DBil, direct bilirubin; eGFR, glomerular filtration rate.
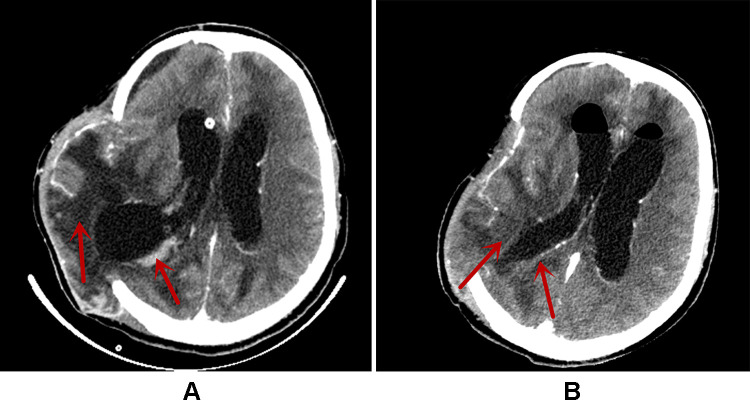
After admission, ceftriaxone (2 g, qd) was commenced as an empirical treatment and an extraventricular drainage was performed the next day. On postoperative Day 2, the patient’s temperature (T), pulse rate (P), and respiratory rate (R) increased to 38.6 °C, 120/min, and 33/min respectively (Table 2), presenting with consciousness coma. Postoperative examination showed that the procalcitonin (PCT) was 22.09 ng/mL, WBC was 7.95×109/L, and N% was 94.5%. An extraventricular drainage yielded clouded cerebrospinal fluid (CSF) with an RBC 230×106/L, nucleated cell count 103×106/L, GLU 0.11 mmol/L, lactate dehydrogenase 549 U/L, lactate 14.32 mmol/L, protein 1115 mg/L and albumin 539 mg/L. The brain computed tomography (CT) scan revealed a localized bulge in the right frontal-temporal lobe, with subdural effusion in the right frontal lobe. Low-density shadows were seen in the frontal lobes, the right basal ganglia, and the temporal lobe. Multiple small nodular enhancements were observed in the right temporal lobe, while there were patchy slightly high-density shadows in the right solid brain posterior horn (Figure 1A). Ceftriaxone was withdrawn and treatment with vancomycin (0.5 g, q6h) and meropenem (1 g, q8h) was commenced due to the aggravation of CNS infection.
Table 2.
Summary of Key Laboratory Data and Antibiotic Treatment, Days 1–22, During Hospitalization
| Day of Admission | Key Laboratory Data | Antimicrobial Regimen |
|---|---|---|
| Day 1 (January 10th) | T 37.6°C Blood routine: WBC 11.87×109/L, N 93.6% |
Ceftriaxone 2 g qd IV |
| Day 4 (January 13th/on postoperative Day 2) | T 38.6°C PCT 22.09 ng/mL Blood routine: WBC 7.95×109/L, N 94.5% Nucleated cells count 103×106/L GLU <0.11 mmol/L CSF total protein: 1115 mg/L CSF albumin: 539mg/L |
Meropenem 1 g q8h IV; Vancomycin 500 mg q6h IV |
| Day 5 (January 14th/on postoperative Day 3) | T 39.6°C CSF bacterial culture: CRAB Blood routine: WBC 7.25×109/L, N 78.8% |
Meropenem 1 g q8h IV; Vancomycin 500 mg q6h IV; Colistin sulfate 50,000 U qd IVT; Colistin sulfate 500,000 U q12h IV |
| Day 10 (January 19th/on postoperative Day 8) | T 38.6°C PCT 4.88 ng/mL Blood routine: WBC 14.84×109/L, N 84.4% CSF: protein positive Nucleated cells count: 2400×106/L GLU: 1.14 mmol/L CSF total protein: 1321 mg/L; CSF albumin: 680 mg/L |
Meropenem 1 g q8h IV; Vancomycin 500 mg q6h IV; Colistin sulfate 100,000 U qd IVT; Colistin sulfate 500,000 U q8h IV |
| Day 15 (January 24th/on postoperative Day 13) | T 38°C PCT 0.26 ng/mL IL-6 13.11 pg/mL CRP 9.4 mg/L CSF bacterial culture: negative Blood routine: WBC 12.16×109/L, N 76.5% CSF: protein weakly positive Nucleated cells count: 150×106/L GLU: 1.9 mmol/L CSF total protein: 1655 mg/L CSF albumin: 962 mg/L |
Regimen maintained |
| Day 19 (January 28th/on postoperative Day 17) | T 37.6°C PCT 0.17 ng/mL CSF bacterial culture: negative Blood routine: WBC 10.7×109/L, N 76.2% CSF: protein weakly positive Nucleated cell count: 187×106/L GLU: 2.45 mmol/L CSF total protein: 1087 mg/L CSF albumin: 668 mg/L |
Regimen maintained |
| Day 22 (January 31st/on postoperative Day 20) | T 37.2°C PCT 0.15 ng/mL CSF bacterial culture: negative Blood routine: WBC 9.65×109/L, N 69.1% CSF: protein weakly positive Nucleated cell count: 12×106/L GLU: 2.31 mmol/L |
Meropenem 1 g q8h IV; Vancomycin 500 mg q6h IV; Colistin sulfate 500,000 U q8h IV |
Figure 1.
The brain CT images during hospitalization. (A) The Brain CT on January 13 (on postoperative Day 2); (B) the Brain CT on January 27 (on postoperative Day 17). The areas indicated by the red arrows in (A and B) are the sites of infection. After treatment, a significant improvement in infection was seen, as shown in (B).
On postoperative Day 3, culture of CSF revealed CRAB, and the broth microdilution method was used for the antimicrobial susceptibility test. Susceptibility results showed resistance to cefoperazone-sulbactam, amikacin, gentamicin, imipenem, meropenem, cefepime, cotrimoxazole, intermediately susceptible to tigecycline, and susceptible to colistin sulfate with a minimum inhibitory concentration (MIC) of 1 mg/L. Subsequently, combination therapy with intravenous (500,000 U, q12h) and intraventricular (50,000 U, qd) colistin sulfate was commenced.
Five days later (on postoperative Day 8), therapeutic drug monitoring (TDM) showed that the concentrations of colistin sulfate in CSF and blood were 2.26 µg/mL and 0.86 µg/mL, respectively. However, the infection was not controlled, the results of laboratory tests were as follows: WBC was 14.84×109/L, N% was 84.8%, and PCT was 4.88 ng/mL. CSF biochemistry: CSF protein was positive, RBC was 2900×106/L, nucleated cell count was 2400×106/L, GLU was 1.14 mmol/L, lactate dehydrogenase was 857 U/L, lactic acid was 10.08 mmol/L, CSF total protein was 1321 mg/L, and CSF albumin was 680 mg/L. Therefore, colistin sulfate was adjusted to 100,000 U qd intraventricularly and 500,000 U q8h intravenously.
On postoperative Day 13, the bacterial culture of CSF became negative, and the blood routine and CSF inflammation indicators were significantly improved. WBC was 12.16×109/L, N% was 76.5%, RBC was 2.9×1012/L, interleukin-6 (IL-6) was 13.11 pg/mL, PCT was 0.26 ng/mL, and C-reactive protein (CRP) was 9.4 mg/L. CSF Biochemistry: CSF protein was qualitatively positive, RBC was 5×106/L, nucleated cell count was 150×106/L, GLU was 1.9 mmol/L, lactate dehydrogenase was 245 U/L, lactic acid was 7.63 mmol/L, CSF total protein was 1655 mg/L, and CSF albumin was 962 mg/L. The trough concentration of colistin sulfate in blood and CSF were 1.41 μg/mL and 4.31μg/mL, respectively. The treatment regimen of IV and IVT colistin sulfate was maintained. Four days later (on postoperative Day 17), the CSF cultures of 3 samples did not isolate A. baumannii. The patient’s temperature returned to normal, and the infection indicators were significantly improved: PCT was 0.17 ng/mL, WBC was 10.7×109/L, and N% was 76.2%. CSF biochemistry: CSF protein was qualitatively weakly positive, RBC was 16×106/L, nucleated cell count was 187×106/L, GLU was 2.45 mmol/L, lactate dehydrogenase was 140 U/L, lactic acid was 6.66 mmol/L, and CSF albumin was 668 mg/L. The follow-up brain CT scan showed that the swelling of the right frontal, parietal, and temporal brain tissues and subdural effusion at the right frontal lobe were improved. A decrease in the size of the low-density shadows was seen which was previously observed in the frontal lobes, right basal ganglia, and temporal lobe. No substantial enhancement in the multiple minor nodular in the right temporal lobe compared with that on postoperative Day 2 (Figure 1B). Therefore, IVT colistin sulfate was discontinued, while IV was maintained. Three days later, the patient’s infection was effectively controlled (Table 2), and he was transferred back to a local hospital for rehabilitation treatment.
Literature Review
Colistin sulfate and CMS are two common, clinically available forms of polymyxin E. CMS is an inactive prodrug that needs to be converted into active colistin (polymyxin E) in vivo. Currently, CMS is widely available in many countries, while colistin sulfate is only clinically available in China, resulting in limited data on efficacy and safety in clinical applications. A systematic literature search in PubMed and relevant biomedical Chinese databases was performed to study in depth the efficacy and safety of colistin in infections. A total of 14 articles that reported different administration routes, treatment regimens, clinical responses, and nephrotoxicity of colistin sulfate were identified (Table 3). Overall, current clinical data showed considerably lower nephrotoxicity of colistin sulfate than those of both polymyxin B and CMS reported in the literature.5 In addition, no adverse events were identified for intrathecal (ITH) and IVT colistin sulfate in the reported cases, but further clinical studies are still needed to comprehensively evaluate the safety of combined IV and IVT/ITH colistin sulfate.
Table 3.
Summary of Clinical Applications of Colistin Sulfate Reported in the Literature
| Medication | Dosage Regimen | Treatment Duration (Days) | Bacteria | Number of Cases | Clinical Response | Nephrotoxicity | References |
|---|---|---|---|---|---|---|---|
| IV+As/IV+ITH | IV 1.5 million U2–3times/dy; As0.25 million Uq12h IVT/ITH 50000 U q24h |
11.63±5.96 | CRO | 42 | 59.52% | None | [4] |
| IV+As | As 0.5–1.0 million U/day | 8.8±1 | MDR-GNB | 9 | 88.90% | None | [6] |
| IV | IV 10,000–20,000 U/kg | - | CRO | 80 | 73.1–94.4% | 6.30% | [7] |
| IV | IV 1.0 million U BID | 14 | MDR-AB | 39 | 87.20% | 5.10% | [8] |
| IV | IV 0.5–1.5 million U/day | 22.2 (10—55) | MDR-GNB | 10 | 75.00% | None | [9] |
| IV | IV 1.0–1.5 million U/day | 14.35 (14~21) | PDR-AB PDR-PA | 15 | 90.00% | 6.70% | [10] |
| IV | IV 1.0–1.5 million U/day | 22.3±6.2 | MDR-GNB | 15 | 73.30% | None | [11] |
| IV | IV 1.5 million U/day | - | CRAB CRKP | 20 | 75.00% | None | [12] |
| IV | IV 1.0–1.5 million U/day | - | CRO | 50 | 58.00% | 6.00% | [13] |
| IV+As /Iv | As 0.25 million U q12h | 14 | MDR-GNB | 116 | 71.00%/41.9% | 16.10%/9.7% | [14] |
| IV | 2.0 million U/day | 35 (23–59) | CRO | 119 | 53.80% | 9.20% | [15] |
| IV+IVT | IV 0.5 million U q8h IVT 50000 U qd | 9 | MDR-AB | 1 | 100.00% | None | [16] |
| IV+ITH | IV 0.75 million U q12h ITH 50000 U qd/qod |
- | CRKP | 1 | 100.00% | None | [17] |
| IV+As | IV 0.75 million U q12h As 0.25 million U q12h |
6 | XDRAB | 1 | 100.00% | None | [18] |
Abbreviations: IV, intravenous; As, aerosolise; ITH, intrathecal; IVT, intraventricular; MDR-GNB, multidrug-resistant gram-negative bacteria; AB, Acinetobacter baumannii; CR, carbapenem-resistant; KP, Klebsiella pneumoniae; PA, Pseudomonas aeruginosa; PDR, pan-drug resistance.
Discussion
According to the recommendations from a domestic expert consensus on the diagnosis and treatment of CNS infections in craniocerebral surgery, ceftriaxone, vancomycin, and meropenem were chosen as initial empirical treatments concerning their penetration into CNS.19 In this case, CSF cultures were positive for CRAB. Results of the antimicrobial susceptibility test showed that the CRAB was resistant to amikacin, cefoperazone sulbactam, intermediately susceptible to tigecycline, and only susceptible to colistin sulfate. However, tigecycline was excluded from consideration due to the intermediate sensitivity, the limited penetration into CSF20, and the severe adverse reaction that occurred in intrathecal injection.21 The monitoring data by the China Antimicrobial Surveillance Network (CHINET) showed that the resistance rate of CRAB to colistin sulfate was very low (around 1.0%).22 Unfortunately, studies revealed that only 5% of intravenous colistin crossed into the CSF, and this penetration ratio did not exceed 25% even in patients with meningitis whose blood–brain barrier was disrupted.23 IVT is a method of injecting a drug directly into the subarachnoid space through a lumbar puncture, allowing the drug to infiltrate the CSF without passing through the blood–brain barrier and reaching its effective concentration quickly.24 The domestic expert consensus on polymyxins recommended that colistin sulfate IV and IVT injection combined with meropenem should be used for the treatment of CNS infections caused by CRO.25 Although CRAB is resistant to meropenem, the combination of meropenem and polymyxin B has a potential synergistic effect on CRAB.26 Meanwhile, meropenem could reach a high concentration in the CSF and the efficacy could be enhanced by prolonging the infusion time.27 After administration of IVT colistin sulfate 50,000 U qd combined with IV 500,000 U q12h for 5 days, the inflammatory indicators of CSF remained abnormal, despite a decrease in PCT. Considering the severity of the infection and the presence of a drainage tube, IVT colistin sulfate was adjusted to 100,000 U qd combined with IV 500,000 U q8h, and treatment was continued for another 9 days. Finally, compared to before, a significant improvement in the infection was seen showing by the subsequent examination results of blood routine and CSF.
In this case, the patient’s infectious characteristics by CSF biochemistry, blood routine, and temperature all showed significant improvements. The infection was solved indicated by the CSF-negative cultures from 3 consecutive samples taken on different days. The patient was transferred back to a local hospital for continued rehabilitation therapy. The duration of antimicrobial treatment after the diagnosis of intracranial infection was 21 days, which was consistent with the treatment course recommended by the multidisciplinary expert consensus on the rational clinical application of polymyxin antibacterial drugs in China.28 In the current case, the duration of intraventricular administration of colistin sulfate was 14 days. Due to the lack of guidelines recommending the optimal duration of intraventricular administration of colistin sulfate, our treatment regimen was decided based on the patient’s clinical response. The treatment duration of IVT and ITH colistin sulfate varied from study to study, while guidelines recommended a duration of 10–21 days for ITH and IVT polymyxin B.16,17,29 A mean treatment duration of 18 days for polymyxin B administered through the IVT or ITH route is recommended by the international consensus guidelines for the optimal use of the polymyxins.30 The optimal treatment duration of IVT colistin sulfate is still unclear, and more clinical studies are required.
Several studies have shown that IVT and ITH CMS and polymyxin B have positive efficacy in the treatment of CNS infections.31–37 However, the clinical data on the treatment regimen of ITH and IVT colistin sulfate are extremely limited. Currently, recommendations on doses of IVT/ITH polymyxins have been reported as polymyxin B (50,000 U/day) and CMS (125,000 U/day).29,30 However, the doses of IVT and ITH colistin sulfate are more clinical and empirical-based. The 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines recommend that according to the ventricular size and daily output of ventricular drainage, dosages, and intervals of intraventricular antimicrobial therapy should be adjusted to achieve CSF antimicrobial concentrations 10–20 times the MIC of the causative microorganism.38 In a study on IVT CMS (100,000 U), the average MIC was 2.91±2.1 μg/mL, and the MIC breakpoint for A. baumannii is 2 μg/mL.39 According to the European Committee for Antimicrobial Susceptibility Testing (EUAST) and the Clinical and Laboratory Standards Institute (CLSI), the expected clinical efficacy might not be achieved at this administration dose. Lu et al reported that a patient with CNS infection caused by multidrug-resistant (MDR) Klebsiella pneumoniae was given 50,000 U ITH colistin sulfate and achieved good clinical efficacy.17 The authors concluded that when administrated at a dose recommended by the international consensus guidelines for optimal use of polymyxins, polymyxin B and colistin sulfate have similar pharmacokinetic characteristics.28 Unfortunately, the authors did not monitor the CSF concentration of colistin sulfate. In other cases, intraventricularly colistin sulfate administration at 50,000 U results in a CSF concentration of 2.478 mg/L.16 However, it was difficult to evaluate the clinical efficacy of these dosing regimens because the treatment duration of colistin sulfate was insufficient due to the dislodgement of the drainage tube. In this case, when administered colistin sulfate in the ventricle at a dose of 50,000 U, the trough concentration in the CSF was 2.26 μg/mL, which did not achieve good clinical efficacy. Therefore, IVT colistin sulfate was modified to 100,000 U qd. After each dose, the drainage tube was clamped for two hours to allow the antimicrobial drug to achieve an equilibrium distribution in the CSF. Five days later, a CSF sample was collected after obtaining the patient’s consent, and the trough concentration of colistin sulfate in CSF increased to 4.31 μg/mL, while the plasma concentration of colistin sulfate was 1.41 μg/mL. A satisfied clinical efficacy was achieved, and the infection was effectively controlled under this dosing regimen.
In our case, unlike other reports, an intraventricular dose of 100,000 U colistin sulfate instead of 50,000 U was given. Surprisingly, no nephrotoxicity or neurotoxicity, such as chemical ventriculitis, chemical meningitis, and seizures, were found during the treatment. In contrast, in a retrospective study on polymyxin B and CMS, toxicity possibly related to the topical use of polymyxins was found in 17 out of 60 patients, of which, the most common toxicity in 12 cases was meningeal irritation.40 Similarly, in a study by Karaiskos et al, adverse events including chemical meningitis/ventriculitis occurred in 11% (9/81) of patients who received IVT/ITH CMS.36 Taking overall, we could infer that IVT colistin sulfate might be a safer option. Individualized dosing and monitoring of drug concentrations in the CSF could optimize colistin sulfate dosing regimens and improve clinical efficacy.
Conclusions
In conclusion, the antimicrobial regimen based on the intravenous and intraventricular colistin sulfate is successful in the treatment of CRAB-caused CNS infection without causing adverse effects such as local chemotaxis inflammation, seizure convulsions, or renal damage. Intraventricular (50,000 U, qd/100,000 U, qd) together with IV (500,000 U, q12h/500,000 U, q8h) colistin sulfate yielded a trough drug concentration in CSF approximately 3 times that in plasma. However, this is only an individual case, and how the size of the ventricular volume and the amount of daily drainage could affect the CSF concentration of colistin sulfate is still unclear. Moreover, extensive clinical studies are needed to further evaluate its efficacy, safety, and treatment duration to optimize the IVT regimen.
Funding Statement
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
CNS, central nervous system; IVT, intraventricular; CRAB, Acinetobacter baumannii; IV, intravenous; CRO, carbapenem-resistant organisms; CMS, colistimethate sodium; T, temperature; P, pulse; R, respiratory rate; WBC, white blood cell count; N%, percentage of neutrophils; RBC, red blood cell count; GLU, glucose; ALT, alanine transaminase; AST, aspartate transaminase; ALB, albumin; TBil, total bilirubin; DBil, direct bilirubin; eGFR, glomerular filtration rate; PCT, procalcitonin; CSF, cerebrospinal fluid; TDM, therapeutic drug monitoring; IL-6, interleukin-6; CRP, C-reactive protein; As, aerosolise; ITH, intrathecal; MDR-GNB, multidrug-resistant gram-negative bacteria; KP, Klebsiella pneumoniae; PA, Pseudomonas aeruginosa; PDR, pan-drug resistance.
Ethical Approval and Informed Consent
Written informed consent was obtained from the patient for the publication of this case. No ethical committee approval was required for this study as the data were analyzed in a retrospective manner.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
- 1.Hernández Ortiz OH, García García HI, Muñoz Ramírez F, et al. Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg. 2018;128(1):262–271. doi: 10.3171/2016.10.JNS16379 [DOI] [PubMed] [Google Scholar]
- 2.Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D. Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infect Drug Resist. 2022;15:697–721. PMID: 35250284; PMCID: PMC8896765. doi: 10.2147/IDR.S326456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang T, Wang X, Wang J, et al. In vitro toxicity study of polymyxin E on renal tubular epithelial cells. Chin J Clin Pharmacol. 2021;37(24):3363–3366+3370. doi: 10.13699/j.cnki.1001-6821.2021.24.015 [DOI] [Google Scholar]
- 4.Yu XB, Zhang XS, Wang YX, et al. Population pharmacokinetics of colistin sulfate in critically ill patients: exposure and clinical efficacy. Front Pharmacol. 2022;13:915958. PMID: 35784679; PMCID: PMC9243584. doi: 10.3389/fphar.2022.915958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akajagbor DS, Wilson SL, Shere-Wolfe KD, Dakum P, Charurat ME, Gilliam BL. Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis. 2013;57(9):1300–1303. PMID: 23840000. doi: 10.1093/cid/cit453 [DOI] [PubMed] [Google Scholar]
- 6.Zhang J-P, Yang X-S, Chen J, Peng Y-Z, Huang Y-S. Clinical evaluation of polymyxin E in the treatment of multi-drug resistant Gram-negative bacillus infection after severe burns. Chin J Burns. 2009;25(5):372–376. doi: 10.3760/cma.j.issn.1009-2587.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 7.Hao M, Yang Y, Guo Y, Wu S, Hu F, Qin X. Combination regimens with colistin sulfate versus colistin sulfate monotherapy in the treatment of infections caused by carbapenem-resistant gram-negative bacilli. Antibiotics. 2022;11(10):1440. PMID: 36290098; PMCID: PMC9598655. doi: 10.3390/antibiotics11101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi LY, Shi LZ, Ma Y. Efficacy of colistin sulfate combined with cefoperazone sodium sulbactam in the treatment of multi-drug resistant Acinetobacter baumannii. J Clin Pathol Sci. 2022;42(3):590–594. doi: 10.3978/j.issn.2095-6959.2022.03.010 [DOI] [Google Scholar]
- 9.Huang J, Mao EQ, Liu W, Qin SH, Tang YQ. Polymyxin E for the treatment of multi-drug resistant Gram-negative bacilli in 10 cases of severe infections. Chin J Infect Chemother. 2008;4:285–288. doi: 10.16718/j.1009-7708.2008.04.006 [DOI] [Google Scholar]
- 10.Liu GP, Shen ZY. Efficacy of polymyxin E in the treatment of severe pulmonary infections caused by pan-drug-resistant immobile bacilli and Pseudomonas aeruginosa. Med Clin Res. 2009;26(2):276–278. [Google Scholar]
- 11.Huang J, Tang YQ, Sun JY. Intravenous colistin sulfate: a rarely used form of polymyxin E for the treatment of severe multidrug-resistant Gram-negative bacterial infections. Scand J Infect Dis. 2010;42(4):260–265. PMID: 20085424. doi: 10.3109/00365540903490018 [DOI] [PubMed] [Google Scholar]
- 12.Xie YL, Jin X, Yan SS, et al. Population pharmacokinetics of intravenous colistin sulfate and dosage optimization in critically ill patients. Front Pharmacol. 2022;13:967412. PMID: 36105229; PMCID: PMC9465641. doi: 10.3389/fphar.2022.967412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin J, Zhu J, Zhu Z, et al. Clinical efficacy and nephrotoxicity of intravenous colistin sulfate in the treatment of carbapenem-resistant gram-negative bacterial infections: a retrospective cohort study. Ann Transl Med. 2022;10(20):1137. PMID: 36388829; PMCID: PMC9652527. doi: 10.21037/atm-22-4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao XL, Tao T, Tang N, et al. Efficacy and safety of adjunctive nebulized colistin sulfate for multidrug-resistant Gram-negative bacteria pneumonia: a retrospective comparative cohort study. Ann Palliat Med. 2022;11(9):2939–2951. PMID: 36217623. doi: 10.21037/apm-22-984 [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Zhong C, Liu Y, et al. Efficacy and safety of polymyxin E sulfate in the treatment of critically ill patients with carbapenem-resistant organism infections. Front Med. 2022;9:1067548. PMID: 36643845; PMCID: PMC9834999. doi: 10.3389/fmed.2022.1067548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu XB, Huang YY, Zhang XS, et al. Intraventricular colistin sulphate as a last resort therapy in a patient with multidrug-resistant Acinetobacter baumannii induced post-neurosurgical ventriculitis. Br J Clin Pharmacol. 2022;88(7):3490–3494. PMID: 35060164. doi: 10.1111/bcp.15238 [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Zhong C, Chen H, Xie X, Lv X. Treatment of central nervous system infection caused by multidrug-resistant Klebsiella pneumoniae with colistin sulfate intravenously and intrathecally: a case report. Pharmaceuticals. 2022;15(12):1482. PMID: 36558933; PMCID: PMC9787966. doi: 10.3390/ph15121482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue X, Zhou T, Wang G, Zhou S. Treatment of pulmonary infection of extensively drug-resistant Acinetobacter baumannii with intravenous colistin sulfate combined with atomization: a case report. Ann Palliat Med. 2021;10(8):9288–9296. PMID: 34488415. doi: 10.21037/apm-21-2112 [DOI] [PubMed] [Google Scholar]
- 19.Neurocritical Care Expert Committee of the Neurosurgeons Branch of the Chinese Physicians Association, Neurosurgery Critical Care Group of the Beijing Medical Association Neurosurgery Branch. Chinese expert consensus on the diagnosis and treatment of central nervous system infections in neurosurgery (2021 edition). Chin J Neurosurg. 2021;37(01):2–15. doi: 10.3760/cma.j.cn112050-20200831-00480. [DOI] [Google Scholar]
- 20.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother. 2006;58(6):1221–1229. PMID: 17012300. doi: 10.1093/jac/dkl403 [DOI] [PubMed] [Google Scholar]
- 21.Li LM, Zheng WJ, Shi SW. Spinal arachnoiditis followed by intrathecal tigecycline therapy for central nervous system infection by extremely drug-resistant Acinetobacter baumannii. J Int Med Res. 2020;48(7):300060520920405. PMID: 32628870; PMCID: PMC7343364. doi: 10.1177/0300060520920405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Ding L, Han R, et al. Current status and trends of antimicrobial resistance among clinical isolates in China: a retrospective study of CHINET from 2018 to 2022. One Health Adv. 2023;8. doi: 10.1186/s44280-023-00009-9 [DOI] [Google Scholar]
- 23.Serafettin Tekgunduz K, Kara M, Caner I, Demirelli Y. Safety and efficacy of intravenous colistin in neonates with culture proven sepsis. Iran J Pediatr. 2015;25(4):e453. PMID: 26396706; PMCID: PMC4575804. doi: 10.5812/ijp.453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziaka M, Markantonis SL, Fousteri M, et al. Combined intravenous and intraventricular administration of colistin methanesulfonate in critically ill patients with central nervous system infection. Antimicrob Agents Chemother. 2013;57(4):1938–1940. PMID: 23335739; PMCID: PMC3623300. doi: 10.1128/AAC.01461-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Committee on Critical Care Medicine, Chinese Society of Research Hospitals, Committee on Evidence-Based and Translational Infectious Diseases, Chinese Society of Research Hospitals. Chinese expert consensus on the clinical application of polymyxin. Chin Crit Care Emerg Med. 2019;31(10):1194–1198. doi: 10.3760/cma.j.issn.2095-4352.2019.10.003. [DOI] [Google Scholar]
- 26.Ni W, Shao X, Di X, Cui J, Wang R, Liu Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: a systematic review and meta-analysis. Int J Antimicrob Agents. 2015;45(1):8–18. PMID: 25465524. doi: 10.1016/j.ijantimicag.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 27.Paul M, Carrara E, Retamar P, et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin Microbiol Infect. 2022;28(4):521–547. PMID: 34923128. doi: 10.1016/j.cmi.2021.11.025 [DOI] [PubMed] [Google Scholar]
- 28.Chinese Association for Medical Education, Chinese Society of Respiratory Diseases, Chinese Society of Intensive Care Medicine, et al.. Multidisciplinary expert consensus on the rational clinical application of polymyxin antibacterial drugs in China. Chin J Tuberc Respir Dis. 2021;44(4):292–310. doi: 10.3760/cma.j.cn112147-20201109-01091. [DOI] [Google Scholar]
- 29.European-Medicines-Agency. Assessment report on polymyxin-based products. Referral under Article 31 of Directive 2001/83/EC. Available from https://www.ema.europa.eu/documents/referral/polymyxin-article-31-referral-assessment-report_en.pdf. Accessed January 24, 2019.
- 30.Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. PMID: 30710469; PMCID: PMC7437259. doi: 10.1002/phar.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Işeri Nepesov M, Ö K, Dinleyici EÇ. Successful intraventricular colistin treatment in resistant Klebsiella pneumoniae meningitis. J Infect Dev Ctries. 2022;16(7):1226–1229. PMID: 35905029. doi: 10.3855/jidc.14096 [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Li X, Li D, Dong X, Chen H. Efficacy and safety of intraventricular polymyxin B plus continuous ventricular drainage for the treatment of intracranial infection caused by drug-resistant Acinetobacter baumannii. Ann Palliat Med. 2022;11(2):490–497. PMID: 35249326. doi: 10.21037/apm-21-3149 [DOI] [PubMed] [Google Scholar]
- 33.Chen F, Deng X, Wang Z, Wang L, Wang K, Gao L. Treatment of severe ventriculitis caused by extensively drug-resistant Acinetobacter baumannii by intraventricular lavage and administration of colistin. Infect Drug Resist. 2019;12:241–247. PMID: 30718963; PMCID: PMC6345184. doi: 10.2147/IDR.S186646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhandari RK, Pandey AK, Shafiq N, et al. Colistin disposition in the cerebrospinal fluid when administered either intravenously alone or with intraventricular/intrathecally in neonates/pediatric patients with culture-proven meningitis. Pediatr Neonatol. 2022;63(2):190–191. PMID: 35148977. doi: 10.1016/j.pedneo.2021.07.012 [DOI] [PubMed] [Google Scholar]
- 35.Chusri S, Sakarunchai I, Kositpantawong N, et al. Outcomes of adjunctive therapy with intrathecal or intraventricular administration of colistin for post-neurosurgical meningitis and ventriculitis due to carbapenem-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(4):646–650. PMID: 29241820. doi: 10.1016/j.ijantimicag.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 36.Karaiskos I, Galani L, Baziaka F, Giamarellou H. Intraventricular and intrathecal colistin as the last therapeutic resort for the treatment of multidrug-resistant and extensively drug-resistant Acinetobacter baumannii ventriculitis and meningitis: a literature review. Int J Antimicrob Agents. 2013;41(6):499–508. PMID: 23507414. doi: 10.1016/j.ijantimicag.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 37.Alnaami I, Alahmari Z. Intrathecal/intraventricular colistin for antibiotic-resistant bacterial CNS infections in pediatric population: a systematic review. Trop Med Infect Dis. 2022;7(3):41. PMID: 35324588; PMCID: PMC8954222. doi: 10.3390/tropicalmed7030041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 infectious diseases society of america’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34–e65. PMID: 28203777; PMCID: PMC5848239. doi: 10.1093/cid/ciw861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni M, Zhao L, Zhang WJ, et al. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration alone in intracranial infections. Int J Antimicrob Agents. 2021;57(3):106281. PMID: 33465459. doi: 10.1016/j.ijantimicag.2021.106281 [DOI] [PubMed] [Google Scholar]
- 40.Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with Gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29(1):9–25. PMID: 17126534. doi: 10.1016/j.ijantimicag.2006.08.024 [DOI] [PubMed] [Google Scholar]