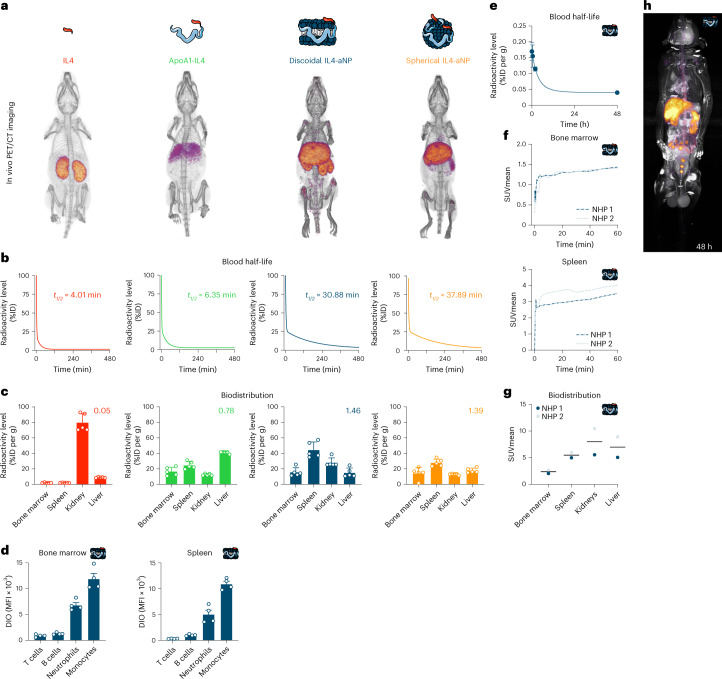
Fig. 5. In vivo pharmacokinetics, biodistribution and safety profile after intravenous injection.
a, PET-CT render at 24 h after injecting 89Zr-labelled constructs. b, 89Zr-labelled construct blood half-life (n = 5, as fitted with a two-phase decay function). ID, injected dose. c, Ex vivo gamma counting of tissues 24 h after 89Zr-labelled construct injection (n = 5), number represents ratio target to clearance organs. d, Cell type-specific biodistribution of DiO-labelled discoid IL4-aNPs in spleen and bone marrow, as measured by flow cytometry. e, 89Zr-IL4-aNP blood half-life in non-human primates. f, Organ SUV mean over time in 89Zr-IL4-aNPs-injected non-human primates (n = 2). g, Organ-specific SUV mean 48 h after 89Zr-IL4-aNPs injection in non-human primates (n = 2). h, PET-MRI scan of non-human primate 48 h after 89Zr-IL4-aNPs injection. Data are presented as mean ± s.d. where appropriate. DIO, 3,3′-dioctadecyloxacarbocyanine perchlorate; MFI, mean fluorescence intensity; NHP, non-human primate.