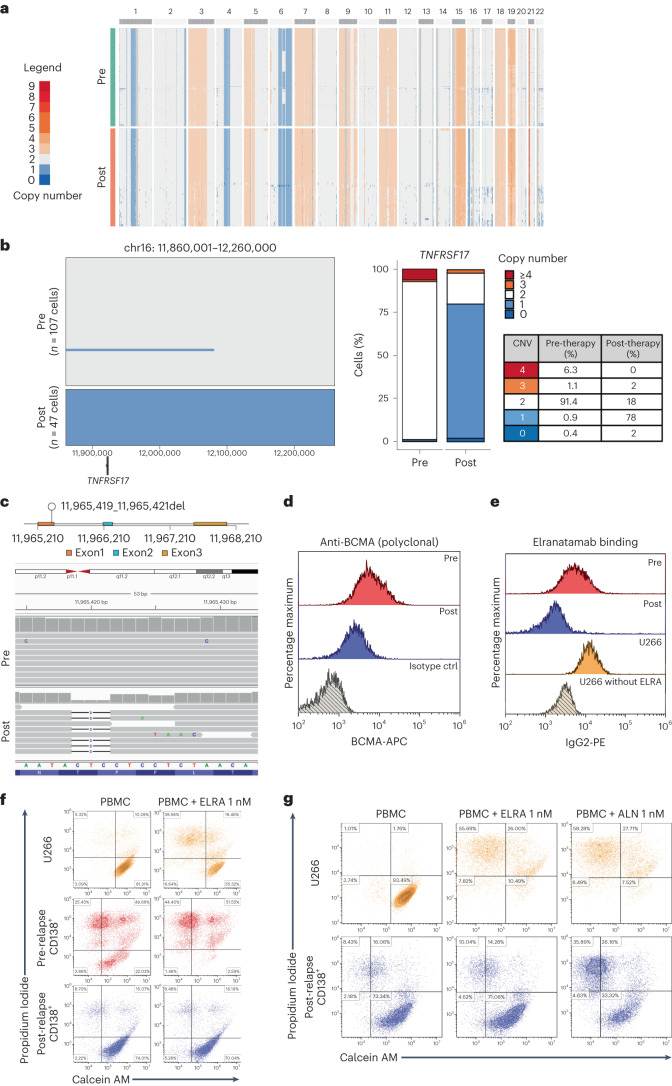
Fig. 5. Monoallelic TNFRSF17 deletion coupled with deletion of Pro34 in the BCMA extracellular domain mediates MM relapse after anti-BCMA TCE therapy in patient MM-15.
a, ScCNV-seq heatmap comparing the CN changes in chromosomes 1–22 in pre-therapy (pre) versus post-relapse (post) CD138+ MM cells. b, Pre-therapy and post-relapse CD138+ CN alteration at TNFRSF17 locus based on scCNV-seq. The barplot and table compare the percentages of cells harboring the CNVs in pre- versus post-CAR T/TCE relapse samples. c, Lollipop plot illustrating the 11965419_11965421del in exon 1 of TNFRSF17 gene. The IGV screenshot illustrates newly detected clonal point mutations in post-relapse CD138+ MM cells. Deletion of 3 bp (chr16: g.11,965,419–11,965,421del) in exon 1 of TNFRSF17 was detected in the post-relapse CD138+ MM sample. This in-frame deletion removes the last two nucleotides of the Thr32 codon and the first nucleotide of the Pro33 codon. This results in retention of Thr32 but deletion of one of the two consecutive proline residues. d, Pre- versus post-relapse CD138+ BCMA protein expression (by polyclonal anti-BCMA antibody) using flow cytometry. e, Pre- or post-relapse patient primary CD138+ cells or U266 MM cell lines incubated with elranatamab (1 nM) followed by secondary flow antibody staining with anti-IgG2 flow antibody. f, Flow cytometry contour with density plot, gated on CTV-positive cells. Patient MM-15’s pre- versus post-relapse CD138+ MM cells or U266 cell line was pre-stained with CTV and co-cultured with patient autologous PBMCs at an effector:target ratio of 10:1, with or without elranatamab (1 nM). The target cell viability is shown at 24 h after co-culture. g, Flow cytometry contour with density plot, gated on CTV-positive cells. Patient’s post-relapse CD138+ MM cell and U266 cell line viability shown at 48 h after co-culture with autologous PBMCs (effector:target = 10:1) with elranatamab 1 nM or alnuctamab 1 nM.