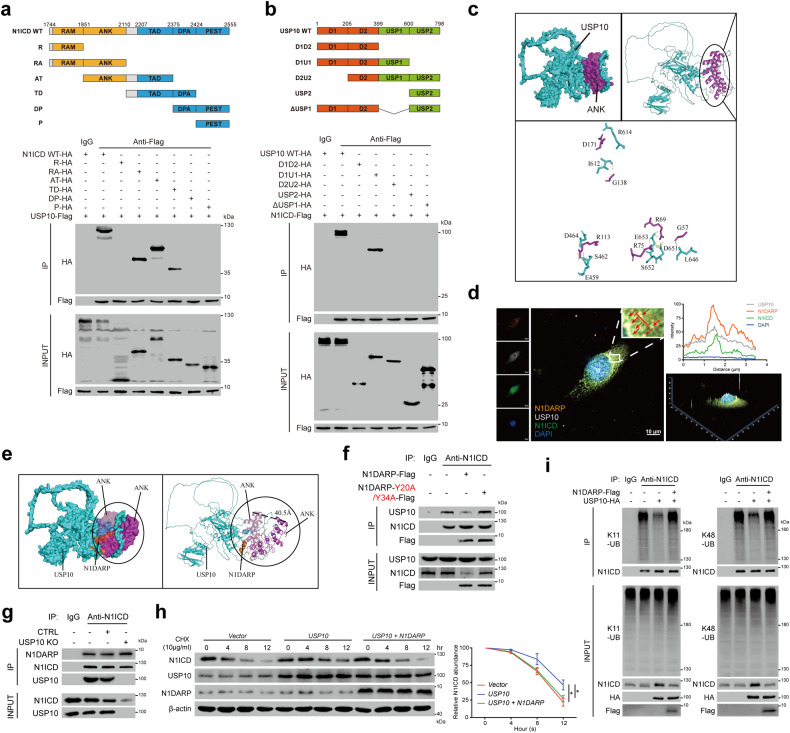
Fig. 7. N1DARP competitively interacts with N1ICD to impinge on USP10-mediated deubiquitination and stabilization of N1ICD.
a Top: strategy for constructing truncated N1ICD plasmids. Bottom: Co-IP followed by western blotting assay for detecting USP10-interacting N1ICD truncations. b Top: strategy for constructing truncated USP10 plasmids. Bottom: Co-IP followed by western blotting assay for detecting N1ICD-interacting USP10 truncations. c Molecular docking simulation of USP10 and ANK domain of N1ICD performed by ClusPro 2.0 and predicted key amino acids mediating USP10–ANK interaction. d Immunofluorescence confocal microscopy using Panc1 confirming co-localization of USP10, N1DARP, and N1ICD in the cytoplasm by detecting simultaneously enhanced fluorescence intensity of these three proteins. The red arrows indicate the co-localization site and the red line represents the fluorescence intensity measured in the right panel. e Molecular docking performed by ClusPro 2.0 simulating the competitive N1DARP-N1ICD binding to obstruct USP10-N1ICD interaction. f Co-IP followed by western blotting assay using Capan1 demonstrating that introduced wild-type N1DARP, not Y20/Y34 mutant, competed with USP10 for interaction with N1ICD. g K11 and K48 polyubiquitination level of N1ICD measured by western blotting assay using K11- or K48-linkage specific polyubiquitination antibody in Capan1 with USP10 overexpression followed by transfection with N1DARP. h Remaining N1ICD at indicated time detected by western blotting assay in Capan1 with overexpressed USP10 followed by N1DARP introduction after treatment with CHX. i Co-IP followed by western blotting assay using Capan1 with USP10 knockout to detect the effect of USP10 on the interaction between N1DARP and N1ICD. The data represent three independent experiments. *P < 0.05 by one-way ANOVA (h). Scale bars, 10 μm (d).