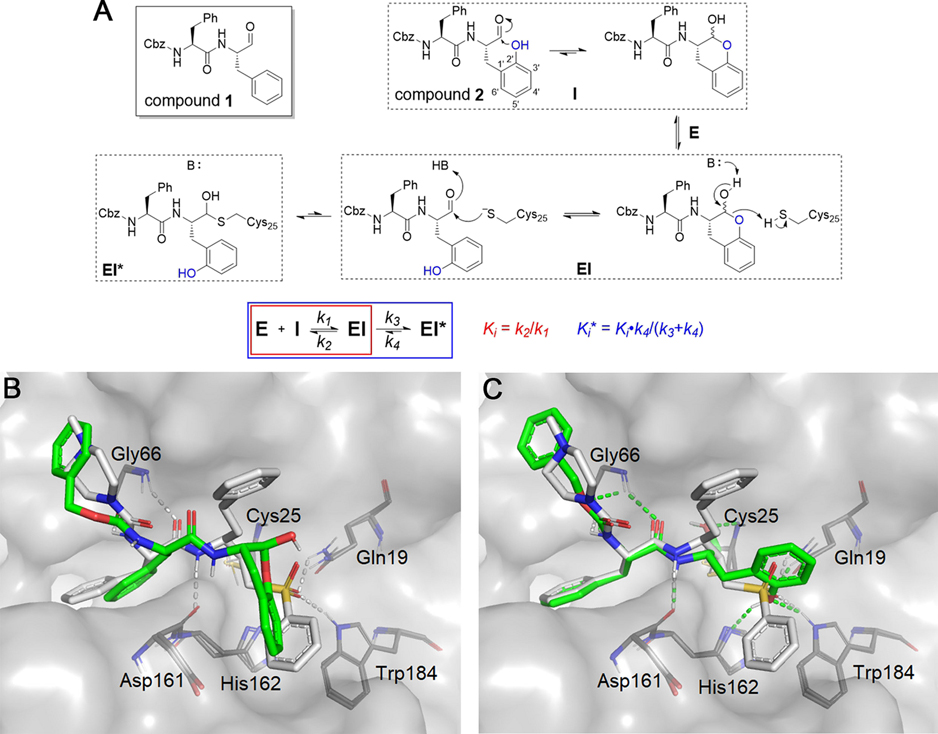
Figure 2.
Rationale for the design of SMAIs. (A) In compound 2, the aldehyde group is expected to be masked by the 2′-phenol group via spontaneous formation of a lactol. It is anticipated that the SMAI will undergo enzyme-catalyzed opening of the lactol ring and subsequently form the hemithioacetal adduct with Cys25. The scheme describes a two-step inhibition mechanism in which rapid formation of an complex precedes isomerization to , which slowly converts back to . (B) Lactol form of 2 (green) noncovalently docked to cruzain. (C) Opened form of 2 covalently docked to form a hemithioacetal with Cys25. Both structures are superimposed with a covalently bound K777 (white) from the crystal structure (PDB ID: 2OZ2).20 Colored dashed lines represent corresponding cruzain–inhibitor interactions.