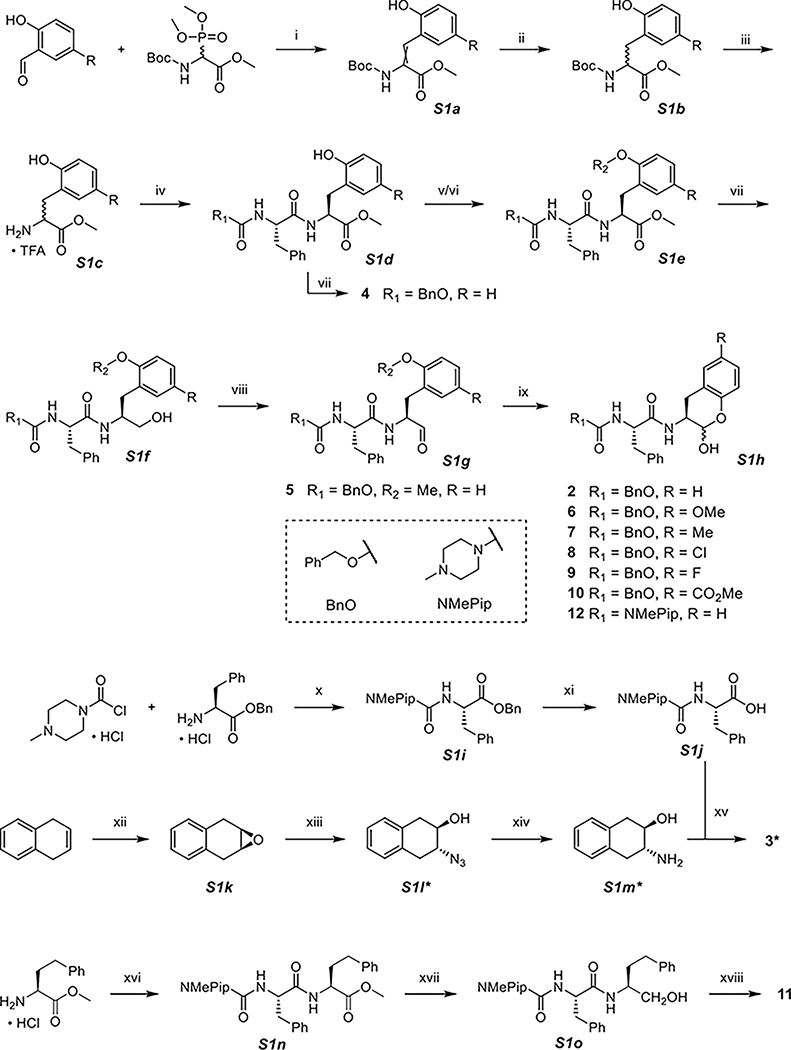
Scheme 1. Synthesis of 2–12a.
a(i) DBU, DCM, (ii) Pd/C, H2, MeOH, (iii) TFA, DCM, (iv) Cbz-Phe-OH or NMePip-Phe-OH (S1j), DIPEA, T3P, DCM, (v) TBSCl, imidazole, DCM, (vi) K2CO3, CH3I, DMF, (vii) NaBH4, MeOH, (viii) Dess-Martin periodinane, NaHCO3, DCM, 0 °C, (ix) TBAF, THF, 0 °C, (x) Et3N, THF, 0 °C, (xi) Pd/C, H2, MeOH, (xii) mCPBA, chloroform, (xiii) NaN3, MeOH/H2O, 60 °C, (xiv) Pd/C, H2, MeOH, (xv) NMePip-Phe-OH (S1j), DIPEA, T3P, DCM, (xvi) NMePip-Phe-OH (S1j), DIPEA, T3P, DCM, (xvii) NaBH4, MeOH, and (xviii) Dess-Martin periodinane, NaHCO3, DCM, 0 °C. *S1l contains two diastereomeric anti products, and only one is drawn here, so do S1m and 3.