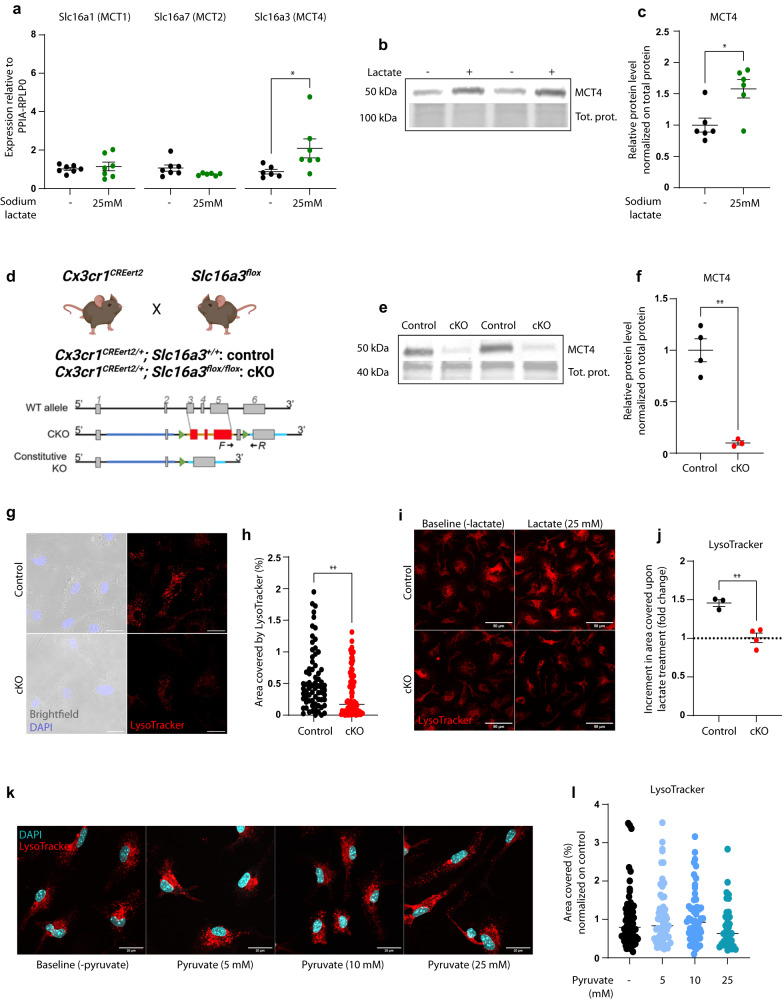
Fig. 2. The lactate transporter MCT4 is upregulated in microglia in response to extracellular lactate and is required for lactate-dependent lysosomal acidification.
a mRNA expression of the lactate transporters Slc16a1, Slc16a7, and Slc16a3 in primary microglia in the presence or absence of 25 mM lactate (24 h treatment). Each data point represents the average of n = 3 technical replicates from N = 7 independent experiments. Two-tailed unpaired t test, *p(Slc16a3) = 0.0481. b Representative western blot of MCT4 protein expression in primary microglia in standard medium (–) or medium containing 25 mM lactate (+, 24 h treatment), and c relative quantification from N = 6 independent experiments. Two-tailed unpaired t test, *p = 0.0104. d Schematic of the breeding strategy for producing Cx3cr1CREERT2;MCT4flox mice and representation of the floxed exons in the MCT4 gene (Slc16a3). Created with BioRender.com. e Representative western blot for MCT4 depletion upon 4-hydroxytamoxifen treatment in primary microglia from control and cKO mice and f relative quantification, normalized on total protein. Data points represent primary microglia prepared from one individual, from N = 4 control and N = 3 cKO. Two-tailed unpaired t test, **p = 0.0011. g Representative confocal z-stack projections of LysoTracker labeling in control and cKO primary microglia and h relative quantification of the area covered by LysoTracker signal per cell. Data points represent individual cells, control: n = 79 cells; cKO: n = 84 cells from N = 2 independent experiments. Two-tailed unpaired t test, **p = 0.0016. Scale bar: 20 µm. i Representative confocal z-stack projections of LysoTracker staining in control and cKO primary microglia, at baseline and after 25 mM lactate exposure, and j signal quantification, displayed as fold change compared to baseline. Data point represent primary microglia prepared from single individuals (control N = 3; cKO N = 4), from three independent experiments. Two-tailed unpaired t test. **p = 0.0025. Scale bar: 50 µm. k Representative confocal z-stack projections of LysoTracker labeling in primary microglia exposed to increasing concentration of pyruvate and l relative quantification of the area covered by LysoTracker signal per cell, normalized to control. Data points represent individual cells from N = 2–3 independent experiments. Baseline (-pyruvate): n = 64 cells, Pyruvate (5 mM): n = 57 cells; Pyruvate (10 mM): n = 57 cells; Pyruvate (25 mM): n = 35 cells. a, c, f, h, j, l Data are represented as mean ± SEM. Source data are provided as a Source Data file.