Abstract
Background
This study determined to probe the potential association between somatic copy number alteration (SCNA) in retinoblastoma (RB) aqueous humour (AH) and pathological high-risk factors, clinical features and previous chemotherapy history.
Methods
Single-centre retrospective cohort study from including 58 AH samples collected from 58 patients diagnosed. Among them, 41 samples were collected after enucleation and 17 samples were collected before intravitreal chemotherapy. SCNAs were accessed by conducting shallow whole-genome sequencing in cell-free (cf) DNA of AH. HRs and ORs were applied to measure risk factors.
Results
RB SCNAs including 1q gain (87%), 2p gain (50%), 6p gain (76%), 16q loss (69%) were frequently detected. Non-classical RB SCNAs in AH including 17q gain (53%), 19q loss (43%), 7q gain (35%) were also commonly observed. 19q loss was significantly more common in patients with cT3c or worse stage than others (p=0.034). 2p gain(p=0.001) and 7q gain(p=0.001) were both more common in patients with primary enucleation than those with previous chemotherapy. Interestingly, both 2p gain (HR=1.933, p=0.027) and 7q gain (HR=2.394, p=0.005) might predict enucleation. Correlation analysis with pathological features among enucleated eyes showed that 19q loss can predict a higher risk for both massive choroid invasion (OR=4.909, p=0.038) and postlaminar optic nerve invasion (OR=4.250, p=0.043).
Discussion
Sequencing of AH cfDNA in RB can provide sufficient in vivo information. 19q loss was a potential signature of advanced cases clinically and pathologically. Repeated sampling from eyes receiving sequential chemotherapy should be conducted to evaluate fluctuation of SCNA in future study.
INTRODUCTION
Retinoblastoma (RB) was the most common paediatric intraocular malignancy,1 and children with RB can lose their vision, eye(s) and life due to this cancer. It has a mortality rate of 12.7% and an enucleation rate of 41.9% in China according to our previous study.2 The mortality rate was 1%–5% in developed countries and as high as 24%–60% in less developed countries in Asia and Africa.1 When the tumour was confined to the eye and the patients received optimised treatment, children’s life can usually be saved.3 However, if the diagnosis or management was delayed, patients may die from metastasis. Unlike most tumours, RB is improper to be biopsied before enucleation due to the risk of tumour seeding and spread out of the eye.4 Additionally, due to the existence of blood–ocular barrier and low overall tumour burden of RB, it was relatively rare to access circulating proteins and cell-free (cf) DNA of intraocular tumour in blood before metastasis happened. Therefore, surrogate liquid biopsy using the aqueous humour (AH) can be carried out in vivo (eg, outside of the setting of enucleation) was supposed to fill this void of preoperative genetic and metabolic profile.
Berry et al innovatively extracted and sequenced cfDNA from the AH,5 which was hypothesised to provide eye-specific tumourous genomic information. Several aspects of cfDNA can be assessed, such as concentration, integrity, mutations and methylation.6 The canonical highly recurrent somatic copy number alteration (SCNA) in RB, including gain of 1q, 2q, 6p and loss of 13q, 16q, were repeatedly identified in cfDNA of AH samples.7 From the aspect of prognosis, the identification of 6p gain in AH was reported to predict higher risk of treatment failure requiring enucleation8 and AH was demonstrated to be more reliable surrogate biopsy than blood9 probably because of the existence of blood–eye barrier. Subsequently, they further demonstrated that biomarkers for poor prognosis can be detected at the time of diagnosis and concentration of AH decreased steadily along with longitudinal intravitreal chemotherapy10 11 correlating with treatment response. In this study, correlation analyses of SCNAs with patients’ clinical features and previous chemotherapy were conducted in order to probe candidate SCNA regarding advanced diseases and chemotherapy.
Even though the potential of AH as surrogate of tumour biopsy has been testified, whether there are biomarkers identifiable in the AH that correlate with pathological high-risk factors has not been fully addressed. Pathological risk factors include: (A) tumour invasion into the anterior chamber, iris, ciliary or sclera, (B) an area of massive posterior uveal invasion ≥3 mm in diameter, (C) postlaminar optic nerve invasion (PLONI) and (D) a combination of any nonmassive posterior uveal invasion (3 mm in diameter) with any degree of non-retrolaminar optic nerve.12 They are widely used to predict metastatic diseases and assist identifying high-risk patients.13 14 However, a worldwide obstacle is that comprehensive pathological report is often inaccessible before enucleation.1 If preoperative prediction for high risk of metastasis can be realised, timely enucleation and proper adjuvant systemic chemotherapy can be given to these at-risk subsets of patients to avoid metastasis and death. Therefore, correlation between SCNAs and pathological high-risk factors were investigated among patients who have received enucleation in this study.
As for extraocular RB, literature is scarce due to the difficulty of biopsy and palliative care setting in late-stage patients. Aschero et al revealed that loss of 11q, gain of 17q and loss of 19q were rarely reported in intraocular cases but more common in extraocular lesions.15
METHODS
Patients and clinical data collection
This is a single-centre, retrospective cohort of 58 patients with RB treated in Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine. Patients were followed up in our clinics. Exclusion criteria are as follows: (1) AH polluted by blood or bacterial infection, (2) AH volume less than 100 μL, (3) total DNA <0.6 μg. The following data were evaluated and carefully documented: (1) demographic characteristics (age, sex, laterality), (2) tumour features under fundus scope (tumour diameter, lesion location, number of lesions, vitreous and/or subretinal seedings), (3) staging (International classifications for retinoblastoma (ICRB) classification and the American Joint Committee on Cancer (AJCC) classification (eighth edition16 17) at the time of sample collection), (4) imaging data (characteristics of orbit, optic nerve or metastasis shown on MRI, CT and ultrasound) and (5) follow-up data (date of recurrence, metastasis or death if happened).2
Pathological data
Eyes enucleated at our centre are routinely evaluated by pathologists in Shanghai Ninth People’s Hospital. Pathological features were documented and cases are stratified according to the AJCC eighth pathological classification. Specifically, pathologists highlighted the pathological high-risk features described.12
Sample collection and processing
Enucleated eyes were sampled immediately after enucleation and preserved eyes were sampled at the time of intravitreal chemotherapy. A paracentesis was conducted with a 32-gauge needle through the cornea to extract 100 μL of AH. The procedure was carried out with extra attention to avoid direct contact between the needles and tumour. AH samples were snap frozen and stored at −80°C sample library of ophthalmology department. The cfDNA of AH samples was extracted using QIAamp Circulating Nucleic Acid Kit (Qiagen), and subsequent DNA library was constructed using the QIAseq UltralowInput LibraryKit (Qiagen). Shallow next-generation sequencing was conducted on Nova HiSeq Platform for a double-end 150 base pair(bp) protocol at an average depth of 0.5×. Tumour DNA fraction of each AH cfDNA was analysed and estimated by ichorCNA software (https://github.com/broadinstitute/ichorCNA),18 which was originally developed to determine tumour DNA fraction for blood-based liquid biopsies.
Data analysis
Statistical analysis was conducted by SPSS software, V.23.0 (IBM). Crosstab analysis was adapted to identify the potential correlation between recurrent SCNA and clinical characteristics. Data are presented as means and SDs/ n (%)/median and IQRs. A p<0.05 by two-sided hypothesis tests was considered significant. Ocular survival and disease-specific survival were depicted by Kaplan-Meier curves. Risk factors of enucleation were identified by Cox hazard proportional models. Risk factors for pathological high-risk features were analysed using logistics regression model. The risk of factors was quantified by HRs, ORs and corresponding CIs.
RESULTS
Clinical and pathological characteristics
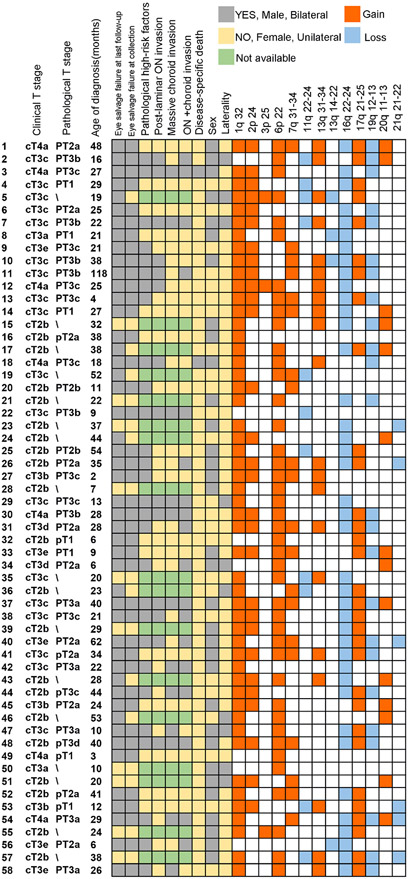
In total, we extracted and sequenced the cfDNA from 58 AH samples of 58 patients. The median follow-up time was 20 months (IQR: 13–29). The median age of diagnosis was 25 months (IQR: 16–37). In this cohort, 48 had unilateral RB and 10 were bilateral cases, from which only 1 eye was included. Forty-one samples were collected immediately after enucleation and the rest 17 were collected at the time of intravitreal chemotherapy. Baseline clinical characteristics of patients were summarised in table 1. Among these 17 patients, 7 of them progressed and required vitrectomy, 3 of whom were eventually enucleated. All patients were diagnosed with T stage worse than cT2b (group D or E). Patients with advanced intraocular tumours (cT3a–cT3e) and extraocular tumour (cT4) took up as much as 63.8% of this cohort. Eventually, two patients died of disease 7 and 14 months after diagnosis, respectively. Both had cT4a RB at the time of diagnosis. At the time the samples were collected, 35 cases had received previous chemotherapy, including any or combination of intravenous, intra-arterial or intravitreal chemotherapy. Among these 35 patients, 31 received previous intravenous chemotherapy (VEC regimen), 23 received previous intra-arterial chemotherapy (melphalan or topotecan) and 14 received previous intravitreal chemotherapy (melphalan or topotecan). Specific combination and times of treatment for each patient were documented in online supplemental table 1. The rest 23 samples were collected after primary enucleation, meaning that they were chemotherapy-free. The pathological reports of the 41 samples collected right after enucleation were centralised reviewed, regarding pathological T stage were presented in table 1. Among these 41 patients, 28 (68%) of them were burdened with pathological high-risk factors. Specifically, 10 (24%) had sclera invasion, 14 (34%) suffered from PLONI, 12 (29%) had massive choroid invasion (diameter >3 mm) and 18 (44%) had combination of any degree of choroid and optic nerve invasion. Two developed brain metastases. The demographic, clinical and pathological characteristics, along with important SCNA information were depicted in figure 1.
Table 1.
Summary of baseline clinical status
| Clinical T stages at the time of sample collection | N (%) Total N=58 |
|---|---|
| cT2b | 21 (36) |
| cT3a | 2 (3) |
| cT3b | 3 (3) |
| cT3c | 18 (31) |
| cT3d | 2 (3) |
| cT3e | 5 (9) |
| cT4a | 7 (12) |
| International classifications for retinoblastoma (ICRB) stages at the time of sample collection |
N (%) Total n=58 |
| D | 15 (26) |
| E | 43 (74) |
| Chemotherapy before sample collection | N (%) Total N=58 |
| Yes | 35 (60) |
| No | 23 (40) |
| Pathological T stage | N (%) Total N=41 |
| pT1 | 7 (17) |
| pT2a | 11 (27) |
| pT2b | 2 (5) |
| pT3a | 5 (12) |
| pT3b | 6 (15) |
| pT3c | 9 (22) |
| pT3d | 1 (2) |
Figure 1.
Clinical, pathological features and SCNA detected in aqueous humour (AH) cell-free (cf) DNA of 58 patients with retinoblastoma. Each row describes the characteristics of one patient. For enucleation status, grey stands for enucleation and yellow stands for eye salvage. For pathological high-risk factors, grey represents positive and yellow represents negative, green indicates that the pathological reports were not available. For sex, grey represents male and yellow represents female. For laterality, grey stands for bilateral disease and yellow stands for unilateral disease. For copy number variation, orange represents gain and blue represents loss. ON, optic nerve; SCNA, somatic copy number variation.
Summary of frequent SCNAs
The canonical RB SCNAs, namely 1q gain, 2p gain, 6p gain and 16q loss, were recurrent RB SCNA detected in all but one AH sample (pt22), among which 1q gain was the most frequent detected in 87% samples, followed by 6p gain (76%) and 16q loss (69%). Recent study discovered novel SCNA including 11q loss (35%), 17q gain (57%) and 19q (30%) loss in tissue of RB.15 In this cohort, 11q loss, 17q gain and 19q loss were detected in 19%, 53% and 43% of AH from patients with RB, which verified the importance of these non-classical SCNA in RB.15
Correlation of SCNAs and clinical features
16q loss had predilection for unilateral cases (36/48) than bilateral cases (4/10) (p=0.055). 19q loss was significantly more commonly observed in patients with cT3c or worse T stage (18/32) than those with better T stage (7/26) (p=0.034).
Correlation of SCNAs and previous chemotherapy
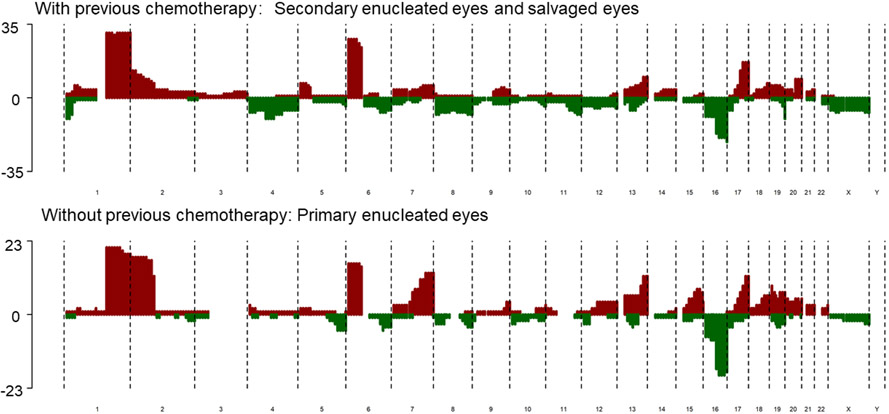
We thoroughly compared the SCNA characteristics of cases with or without chemotherapy before sample collection (figure 2, (online supplemental table 2)). We found that 2p gain was significantly more common in chemotherapy-free samples collected after primary enucleation (18/23) than in patients with chemotherapy history (11/35) (p=0.001). Specifically, among the subset with chemotherapy, the frequency of 2p gain was higher in samples of secondary enucleation (6/17) than those preserved (5/18). Another SCNA that was more frequently observed in primary enucleated eyes was 7q gain (9/23 vs 6/35, p=0.001). In addition, the ratio of both 2p gain (18/24 vs 6/17, p=0.023) and 7q gain (14/24 vs 3/17, p=0.012) were higher in primary enucleated eyes than in secondary enucleated eyes (online supplemental table 3). Correlation of different chemotherapy and 2p gain and 7q gain was summarised in online supplemental table 4. Results showed that 2p gain or 7q gain were less frequent in cases with previous IVC and/or IAC history. However, only 7q gain was significantly less with previous intravitreal chemotherapy.
Figure 2.
Composite SCNA profile of cfDNA sampled from eyes with previous chemotherapy and primary enucleated eyes. cfDNA, cell-free DNA; SCNA, somatic copy number alteration.
We further discover the tendency of coexistence of 2p gain and 7q gain. In total, there were 15 patients who had both 2p gain and 7q gain (p=0.012) among the 20 patients with 7q gain and 29 patients with 2p gain. Of note, among the 35 patients with previous chemotherapy, only 2 had both 2p gain and 7q gain. However, among the 23 patients receiving primary enucleation, 12 had coexistence of 2p and 7q gain (p<0.001).
Correlation of SCNAs and eye salvage
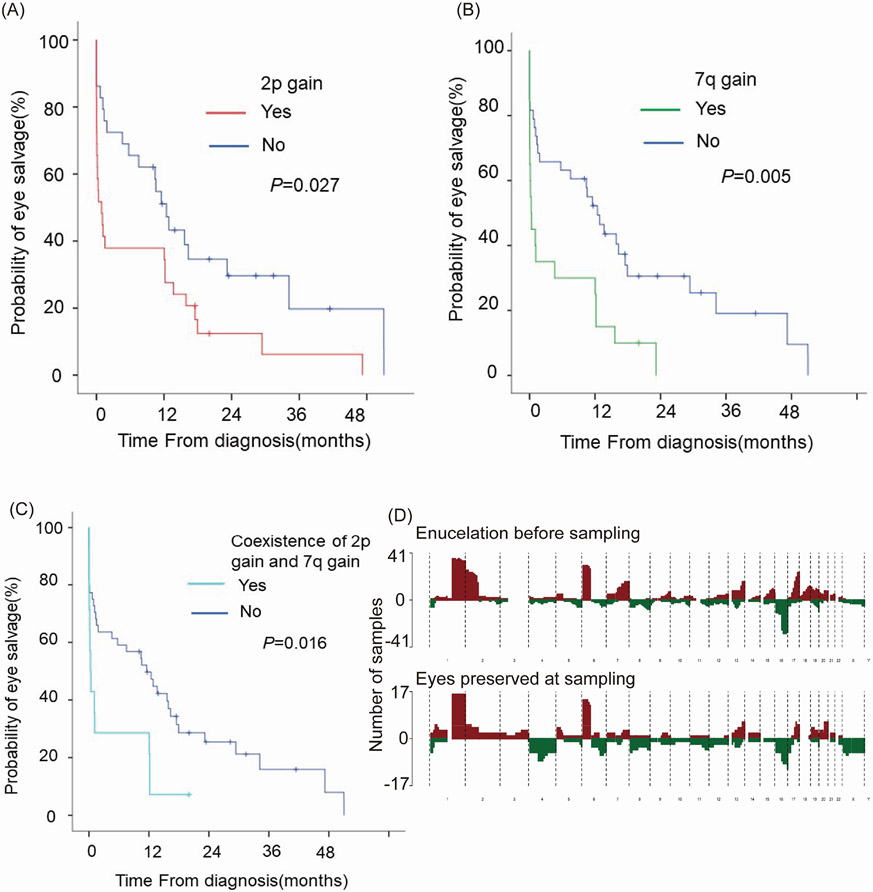
To determine the possible risk SCNA for eye salvage failure, we conducted unifactorial and multifactorial cox hazard analysis in this cohort of 58 patients. Among the common RB SCNA mentioned above, cases with 2p gain (HR 1.933, 95% CI 1.078 to 3.466, p=0.027, figure 3A) or 7q gain (HR 2.394, 95% CI 1.303 to 4.399, p=0.005, figure 3B) may be predictors for higher risk of eye salvage failure. Because the tendency of coexistence of 2p gain and 7q gain, we looked into the patients with both 2p gain and 7q gain, and found that they had a 2.282-fold increased risk of eye salvage failure (95% CI 1.168 to 4.459, p=0.016, figure 3C). By further including these two CNAs into one multifactorial cox model, we found that 7q gain still might act as a risk for eye salvage failure (HR 2.072, 95% CI 1.095 to 3.922, p=0.025). The composite SCNA profiles of cfDNA collected from the AH of enucleated and salvaged eyes at the time of sampling were summarised in figure 3D.
Figure 3.
Association of 2p gain and 7q gain with eye salvage failure (A) Kaplan-Meier plot of ocular survival stratified by 2p gain (B) Kaplan-Meier plot of ocular survival stratified by 7q gain (C) Kaplan-Meier plot of ocular survival stratified by coexistence of 2p gain and 7q gain (D) Composite SCNA profile of cfDNA collected from the AH of enucleated and salvaged eyes at the time of sampling, red represents gain and green represents loss. cfDNA, cell-free DNA; SCNA, somatic copy number alteration.
However, in order to better verify the correlation of these SCNAs and enucleation, sequential sample collection and globe survival analysis should be conducted among patients who initially received eye preserving therapies.
Correlation of SCNAs and pathological features
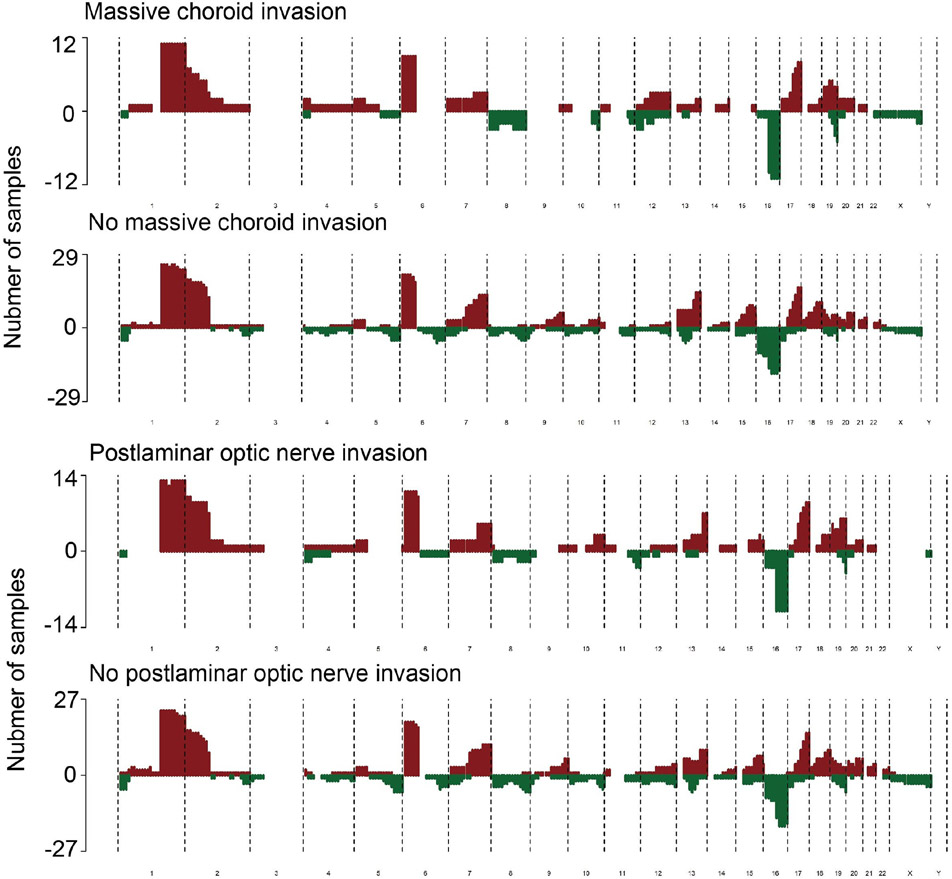
Next, we conducted correlation analysis of pathological features and SCNAs in the 41 patients who received enucleation. As the AH has been proven as an effective alternative biopsy material, using AH to predict pathological high-risk factors is of great interest in real-world clinical scenarios. In order to identify potential CNA associated with pathological high-risk factors, we compared the CNA of patients with or without massive posterior uveal invasion, postlaminar optic nerve invasion, sclera invasion or a combination of any degree of uveal invasion with any degree of optic nerve invasion. 19q loss took up 75% (9/12) in patients with massive choroid invasion, almost twice as much as that among patients without (11/29) (p=0.043) (figure 4A). By logistics regression, we found that patients with 19q loss had 4.909-fold risk (95% CI 1.088 to 22.148, p=0.038) of massive choroid invasion. All patients with massive choroid invasion had either 19q loss or eyeball wall enhancement on MRI (p=0.001). Interestingly, 19q loss was also much more commonly observed in cases with postlaminar optic nerve invasion (10/14) than those without (10/27) (figure 4B). By logistics regression, 19q loss also predicted a higher risk of postlaminar optic nerve invasion (OR 4.250, 95% CI 1.050 to 17.202, p=0.043). Coincidently, these patients with postlaminar optic nerve invasion were also all the patients with extraocular extension in this cohort. These above evidences, along with the fact of higher proportion of 19q loss in cases of cT3c or worse stage, suggested the potential role that 19q loss may play in the invasion of RB. No significant correlation was identified between other recurrent SCNAs and different pathological high-risk factors. The logistics models for different pathological factors were summarised in online supplemental table 5.
Figure 4.
19q loss increased the risk of specific pathological high-risk factors (A) Composite SCNA profile of cfDNA collected from the AH of enucleated eyes with or without massive choroid invasion (B) Composite SCNA profile of cfDNA collected from the AH of enucleated eyes with or without postlaminar ON invasion. AH, aqueous humour; cfDNA, cell-free DNA, ON, optic nerve; SCNA, somatic copy number alteration.
DISCUSSION
Alternative biopsy of AH in RB has gained increased utility since first introduction in 2017. Compared with traditional tumour biopsy, AH biopsy can be done preoperatively, successively to monitor dynamic changes of the tumour progression and reaction to therapies. Information of in vivo molecular changes can provide the ophthalmologists with sufficient evidence to tailor precision treatment. From the aspect of financial burden, it is much more affordable, costing only one-sixth of tumour biopsy.
Majority of included eyes in this research were at advanced groups. Up to date, research on cfDNA of RB AH was mostly restricted to advanced groups. A previous research on AH cfDNA included three patients with group B/C RB. The concentration of eight AH samples was tested and they ranged from 0.084 to 56 ng/μL. Concentration of cfDNA in the AH of group B eye was 0.18 ng/μL, yet no RB SCNA was detected. Concentration in the two group C eyes was not reported, however, RB SCNAs were detected in both eyes. These results suggested that AH of eyes with smaller tumour (group B/C) could also be used for cfDNA sequencing. However, extensive research on smaller tumour should be carried out to test the efficiency of using AH as surrogate biopsy.
Previous literature regarding non-classical SCNAs 7q gain, 11q loss, 17q gain and 19q loss in AH is scarce. 19q loss was described as a secondary alteration in the AH of one aggressive eye.11
In this study, the most common SCNAs in this study including 1q gain (87%), 6p gain (76%), 16q loss (69%) and 2p gain (50%) were recurrently reported in numerous studies on RB. The frequency of 1q gain was much higher than that in an US cohort of 26 patients (38%).8 6p gain was also more common (76% vs 62%) with a higher median amplitude of 4.0. 6p gain was previously established as a predictor for enucleation as well as aggressive histopathological features predicting microinvasion,19 however, in this cohort, 76% of the patients had 6p gain and statistical difference was not identified. This could be explained by the fact that this cohort only contained patients diagnosed with T2b or higher stage (74% were diagnosed as group E). When correlating with clinical features, 16p loss was found to be more frequent in our patients with unilateral disease than bilateral disease. 16p involved CDH1120 and RBL2,21 22 coded for protein p130, which were both recognised as key regulators of RB. Though 13q14 loss was relatively low in this cohort, 13q31-34 gain was detected in 38% cases, which indicated instability of the 13q segment. In addition, 19q loss is associated with worse the American Joint Committee on Cancer (AJCC) staging.
In correlation analysis between SCNAs and previous chemotherapy history, 2p gain, a well-stablished RB,23 was found to be more common in samples from primary enucleated eyes than samples with chemotherapy (78% vs 31%), which was in accordance with another study conducted in tumour tissue (65% vs 50%).15 Gain of 7q was more prevalent in advanced stage neuroblastoma, but the report of 7q gain in RB was limited.24 25 A previous meta-analysis trying to identify driver genes in RB suggested that NUP205 may contribute to tumour progression, yet further validation was not conducted.26 It was hypothesised that RB went through subclone selection during tumour progression and chemotherapy, dynamic SCNA profiling may further reflect this process.27 However, to establish the relation between these two SCNAs and response to chemotherapy, before-and-after study in same patients undergoing sequencing chemotherapy should be considered.
17q loss has been reported in two cases with orbital and cervical lymph node involvement, co-occurred with 16q loss and MYCN amplification.28 An important associative gene located at 17q was CDK3, which promotes entry into S phase and phosphorylates the RB1 protein to induce exit from phase G0.29
Investigation of pathological features and extraocular extension highlighted the importance of 19q loss. Specifically, 19q loss was found to increase the risk of both massive choroid invasion and postlaminar ON invasion by approximately fourfold. The most involved segment was 19q13.3–13.4. 19q loss was commonly detected in glioma of nervous system, especially oligodendroglioma.30 Moreover, loss of heterozygosity at 19q13.3 was reported to correlated with locally aggressive neuroblastoma.31 Historically, RB, neuroblastoma and brain tumours have been sometimes studied integratedly as tumours of neuroectodermal origin.32 Therefore, it is understandable that 19q loss may play a significant role in RB as in central nervous system cancers. Dysregulation AKT2, one of AKT kinase located at 19q13.3, was potentially correlated with tumour progression. In addition, the PI3k-Akt pathway was found to deregulated in RB organoid.33
Limitations
This study compared samples collected from patients who received primary enucleation and patients who have received previous chemotherapy. To investigate the fluctuation of SCNAs in response to selective pressure like chemotherapy, samples should be collected from identical patients during sequential chemotherapy.19 34 Further research on baseline and evolution of SCNA from patients receiving sequential chemotherapy should be carried out.
Supplementary Material
WHAT IS ALREADY KNOWN ON THIS TOPIC
⇒ Aqueous humour (AH) has been testified to be effective alternative biopsy material for retinoblastoma. Previous study has associated AH somatic copy number alteration with enucleation, yet their correlation with pathological and clinical factors needs to be further explored.
WHAT THIS STUDY ADDS
⇒ 2p gain and 7q gain were more common in primary enucleated eyes and 19q loss was a potential signature of advanced cases clinically and pathologically.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
⇒ Biopsy of AH can guide precise decision of enucleation and chemotherapy.
Acknowledgements
We would like to thank Dr. Jia Jia and Li Zhang from Fulgent Technologies Inc for their kind advice on bioinformatic analysis.
Funding
This work was supported by National Natural Science Foundation of China (grant 81570884, 81872339, 82272642), Shanghai Municipal Science and Technology Major Project (17JC1420100), Shanghai Municipal Science and Technology Major Project (19JC1410202), Shanghai Science and Technology Development Funds (Grant 17DZ2260100, 19QA1405100), Shanghai Youth Top-notch Talent Support Programme and Shanghai Ninth People’s Hospital Excellent Youth Fund Programme (JYYQ003).
Footnotes
Disclaimer The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests None declared.
Patient consent for publication Consent obtained from parent(s)/guardian(s).
Ethics approval This study involves human participants and was approved by the research ethics committees of Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine ( approval ID: SH9H-2019-T185-2). Participants gave informed consent to participate in the study before taking part.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
REFERENCES
- 1.Dimaras H, Kimani K, Dimba EAO, et al. Retinoblastoma. Lancet 2012:379:1436–46. [DOI] [PubMed] [Google Scholar]
- 2.Luo Y, Zhou C, He F, et al. Contemporary update of retinoblastoma in china: three-decade changes in epidemiology, clinical features, treatments, and outcomes. Am J Ophthalmol 2022:236:S0002-9394(21)00498-0:193–203.:. [DOI] [PubMed] [Google Scholar]
- 3.Munier FL, Beck-Popovic M, Chantada GL, et al. Conservative management of retinoblastoma: challenging orthodoxy without compromising the state of metastatic grace. “alive, with good vision and no comorbidity.” Prog Retin Eye Res 2019:73:S1350-9462(18)30073-9:100764.:. [DOI] [PubMed] [Google Scholar]
- 4.Karcioglu ZA, Gordon RA, Karcioglu GL. Tumor seeding in ocular fine needle aspiration biopsy. Ophthalmology 1985:92:1763–7. [DOI] [PubMed] [Google Scholar]
- 5.Berry JL, Xu L, Murphree AL, et al. Potential of aqueous humor as a surrogate tumor biopsy for retinoblastoma. JAMA Ophthalmol 2017:135:1221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valpione S, Gremel G, Mundra P, et al. Plasma total cell-free DNA (cfdna) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur J Cancer 2018:88:S0959-8049(17)31369-2:1–9.:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim ME, Polski A, Xu L, et al. Comprehensive somatic copy number analysis using aqueous humor liquid biopsy for retinoblastoma. Cancers 2021:13:3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry JL, Xu L, Kooi I, et al. Genomic cfdna analysis of aqueous humor in retinoblastoma predicts eye salvage: the surrogate tumor biopsy for retinoblastoma. Mol Cancer Res 2018:16:1701–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry JL, Xu L, Polski A, et al. Aqueous humor is superior to blood as a liquid biopsy for retinoblastoma. Ophthalmology 2020:127:S0161-6420(19)32184-0:552–4.:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu L, Kim ME, Polski A, et al. Establishing the clinical utility of ctDNA analysis for diagnosis, prognosis, and treatment monitoring of retinoblastoma: the aqueous humor liquid biopsy. Cancers (Basel) 2021:13:1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polski A, Xu L, Prabakar RK, et al. Cell-Free DNA tumor fraction in the aqueous humor is associated with therapeutic response in retinoblastoma patients. Trans Vis Sci Tech 2020:9:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan RC, Qaddoumi I, Billups CA, et al. Comparison of high-risk histopathological features in eyes with primary or secondary enucleation for retinoblastoma. Br J Ophthalmol 2015:99:1366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaliki S, Shields CL, Shah SU, et al. Postenucleation adjuvant chemotherapy with vincristine, etoposide, and carboplatin for the treatment of high-risk retinoblastoma. Arch Ophthalmol 2011:129:1422–7. [DOI] [PubMed] [Google Scholar]
- 14.Honavar SG, Singh AD, Shields CL, et al. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol 2002:120:923–31. [DOI] [PubMed] [Google Scholar]
- 15.Aschero R, Francis JH, Ganiewich D, et al. Recurrent somatic chromosomal abnormalities in relapsed extraocular retinoblastoma. Cancers (Basel) 2021:13:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomar AS, Finger PT, Gallie B, et al. A multicenter, international collaborative study for American joint Committee on cancer staging of retinoblastoma: Part II: treatment success and globe salvage. Ophthalmology 2020:127:S0161-6420(20)30524-8:1733–46.:. [DOI] [PubMed] [Google Scholar]
- 17.Grubbs EG,Williams MD, Scheet P, et al. Role of CDKN2C copy number in sporadic medullary thyroid carcinoma. Thyroid 2016:26:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adalsteinsson VA, Ha G, Freeman SS, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun 2017:8:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xi W, Zhou C, Xu F, et al. Molecular evolutionary process of advanced gastric cancer during sequential chemotherapy detected by circulating tumor DNA. J Transl Med 2022:20:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchong MN, Chen D, Corson TW, et al. Minimal 16q genomic loss implicates cadherin-11 in retinoblastoma. Mol Cancer Res 2004:2:495–503. [PubMed] [Google Scholar]
- 21.Priya K, Jada SR, Quah BL, et al. High incidence of allelic loss at 16q12.2 region spanning RBL2/p130 gene in retinoblastoma. Cancer Biol Ther 2009:8:714–7. [DOI] [PubMed] [Google Scholar]
- 22.Kooi IE, van SE, MacPherson D, et al. Genomic landscape of retinoblastoma in rb(−/−) p130(−/−) mice resembles human retinoblastoma. Genes Chromosomes Cancer 2017:56:231–42. [DOI] [PubMed] [Google Scholar]
- 23.Rushlow DE, Mol BM, Kennett JY, et al. Characterisation of retinoblastomas without Rb1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol 2013:14:S1470-2045(13)70045-7:327–34.:. [DOI] [PubMed] [Google Scholar]
- 24.Stallings RL, Howard J, Dunlop A, et al. Are gains of chromosomal regions 7q and 11p important abnormalities in neuroblastoma? Cancer Genet Cytogenet 2003:140:133–7. [DOI] [PubMed] [Google Scholar]
- 25.Vandesompele J, Speleman F, Van Roy N, et al. Multicentre analysis of patterns of DNA gains and losses in 204 neuroblastoma tumors: how many genetic subgroups are there? Med Pediatr Oncol 2001:36:5–10. [DOI] [PubMed] [Google Scholar]
- 26.Kooi IE, Mol BM, Massink MPG, et al. A meta-analysis of retinoblastoma copy numbers refines the list of possible driver genes involved in tumor progression. PLoS One 2016:11 :e0153323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kooi IE, Mol BM, Massink MPG, et al. Somatic genomic alterations in retinoblastoma beyond Rb1 are rare and limited to copy number changes. Sci Rep 2016:6:25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zugbi S, Ganiewich D, Bhattacharyya A, et al. Clinical, genomic, and pharmacological study of MYCN-amplified Rb1 wild-type metastatic retinoblastoma. Cancers 2020:12:2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren S, Rollins BJ. Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 2004:117:239–51. [DOI] [PubMed] [Google Scholar]
- 30.Wesseling P, Capper D. Who 2016 classification of gliomas. Neuropathol Appl Neurobiol 2018:44:139–50. 10.1111/nan.12432 Available: http://doi.wiley.com/10.1111/nan.2018.44.issue-2 [DOI] [PubMed] [Google Scholar]
- 31.Mora J, Cheung NK, Chen L, et al. Loss of heterozygosity at 19q13.3 is associated with locally aggressive neuroblastoma. Clin Cancer Res 2001:7:1358–61. [PubMed] [Google Scholar]
- 32.Bostrom B, Mirkin BL. Elevation of cerebrospinal fluid catecholamine metabolites in patients with intracranial tumors of neuroectodermal origin. J Clin Oncol 1987:5:1090–7. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Zhang Y, Zhang Y-Y, et al. Human embryonic stem cell-derived organoid retinoblastoma reveals a cancerous origin. Proc Natl Acad Sci U S A 2020:117:33628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehi S, Kabeer F, Ceglia N, et al. Clonal fitness inferred from time-series modelling of single-cell cancer genomes. Nature 2021:595:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.