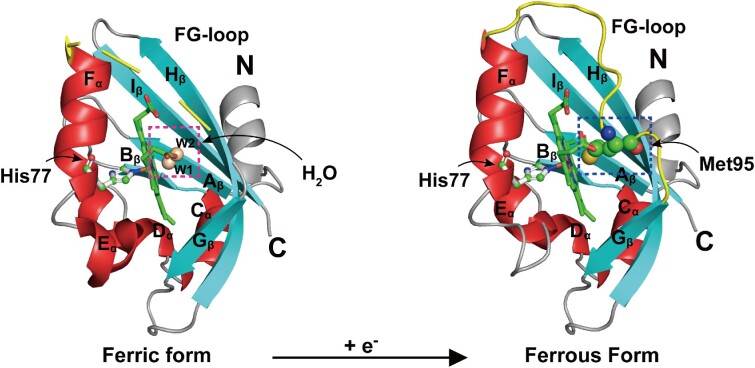
Figure 2.
Redox-induced changes at the distal heme site of EcDosP. EcDosP is O2-dependent and its activity is regulated by the transition between the ferric form (PDB entry:1V9Y) (Kurokawa et al. 2004) and ferrous form (PDB entry:1V9Z) (Kurokawa et al. 2004) of the heme-PAS domain, a process accompanied by the change of distal axial ligands. When the heme-PAS domain is in the ferric form, the distal axial ligand of its complex is a water molecule W1, stabilized by another water molecule W2 (marked by the magenta dashed box); when it is reduced to the ferrous form, the distal axial ligand of its complex is changed to Met95 (marked by the blue dashed box) of the FG-loop (shown in yellow). Also indicated is the iron-binding His77.