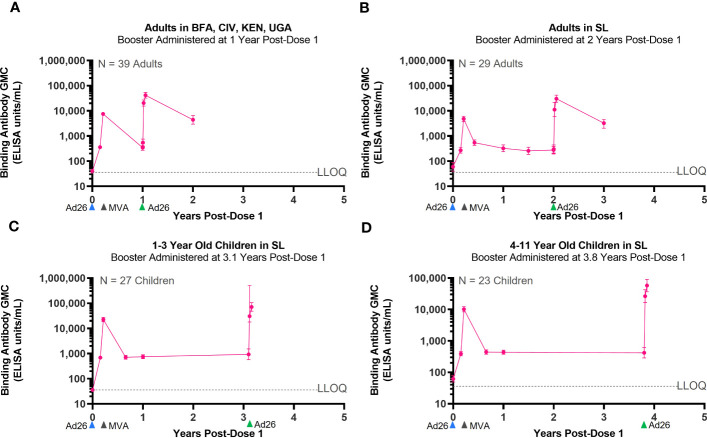
Figure 3.
Persistence of the primary immune response after vaccination with the Ad26.ZEBOV, MVA-BN-Filo vaccine regimen and activation of an immune memory response after administration of an Ad26.ZEBOV booster dose. EBOV GP-binding antibody GMCs in EU/mL at various time points, with accompanying 95% CIs, are depicted. Ad26.ZEBOV booster dose administered between 1 year and 3.8 years post-dose 1 in adults (A, B) and children (C, D). Samples were analyzed following standard operating procedure at Q2 Solutions using the FANG ELISA, and a single reportable value for each sample at each time point was uploaded for statistical analysis. The horizontal dashed lines indicate the FANG ELISA lower limit of quantification of 36.11 EU/mL. The blue arrowheads below the x-axis indicate the timing of administration of the Ad26.ZEBOV vaccine doses, the black arrowheads indicate timing of administration of the MVA-BN-Filo vaccine dose, and the green arrowheads indicate timing of administration of the Ad26.ZEBOV booster dose. CI, confidence interval; EBOV GP, Ebola virus glycoprotein; ELISA, enzyme-linked immunosorbent assay; EU, ELISA unit; mL, milliliter; FANG, Filovirus Animal Nonclinical Group; GMC, geometric mean concentration; Ad26, Ad26.ZEBOV; MVA, MVA-BN-Filo; Yrs, years; BFA, Burkina Faso; CIV, Côte d’Ivoire; KEN, Kenya; UGA, Uganda; GNA, Guinea; LIB, Liberia; MAL, Mali; SL, Sierra Leone. N is the number of participants with data at pre-booster baseline.