Figure EV4. Resolution of HMCES‐DPCs during replication‐coupled ICL repair. Related to Fig 5 .
-
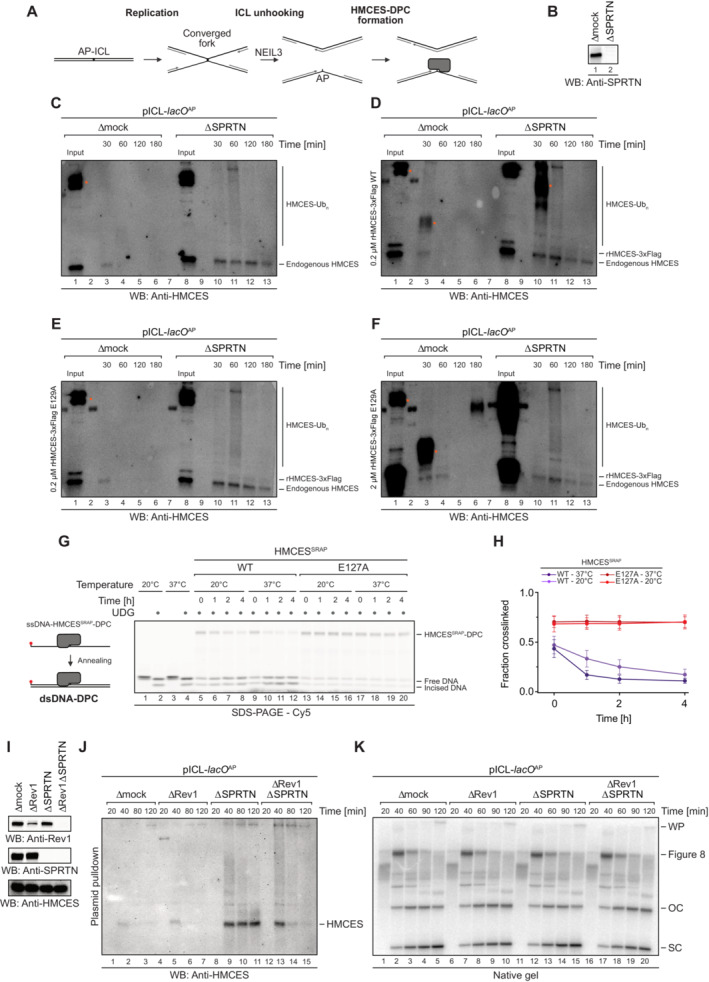
ASchematic depiction of the NEIL3‐dependent repair of an AP‐ICL, a lesion that forms when an AP site reacts with a nucleobase of the opposing DNA strand forming a covalent crosslink (Price et al, 2014). In Xenopus egg extracts, such crosslinks are primarily unhooked by the NEIL3 glycosylase (Semlow et al, 2016), which yields an AP site leading to formation of an HMCES‐DPC.
-
B–FIn the absence of SPRTN, the intact HMCES‐DPC is presumably bypassed by TLS and transferred into dsDNA. To test whether this triggers autorelease, we analysed the stability of DPCs formed by wild‐type and E129A‐mutated Xenopus laevis rHMCES‐3xFlag proteins during ICL repair in egg extract. pICL‐lacO AP was replicated in mock‐ or SPRTN‐depleted extracts (B) supplemented with WT or E129A rHMCES‐3xFlag. At the time points indicated, plasmid was recovered under stringent conditions, the DNA was digested and released proteins were separated by SDS–PAGE. HMCES‐DPCs were detected using an antibody raised against the SRAP domain that permits simultaneous monitoring of endogenous HMCES protein and the recombinant 3xFlag‐tagged HMCES (which migrates slower during SDS–PAGE due to the 3xFlag). In this experimental setup, the endogenous protein serves as a control for the effects of SPRTN depletion and autorelease. Like the endogenous HMCES (C), both WT (D) and E129A‐mutated rHMCES‐3xFlag (E) were stabilized by SPRTN depletion, implying that proteolysis is the dominant mechanism for removing HMCES‐DPC under these conditions. However, it was challenging to assess the relative behaviour of tagged WT and mutant protein because DPCs formed by the wild‐type recombinant protein (like those formed by the endogenous protein) are resolved slowly in SPRTN‐depleted extract (on the timescale of hours, somewhat slower than the timescale for observed for reversal in vitro). Additionally, the E129A‐mutated recombinant flag‐tagged protein crosslinked less efficiently than endogenous HMCES, making it difficult to detect even when present in large excess (F). We are, therefore, unable to determine from these data whether HMCES‐DPC reversal occurs during ICL repair in egg extract under the conditions tested. Orange dots denote non‐specific bands or bands corresponding to contaminating IgG.
-
GOne explanation for the discrepancy in the degree of reversal observed between the in vitro reconstitution and egg extract systems could be that the in vitro reactions were all performed at 37°C, while replication in egg extracts must be performed at 20°C. Therefore, we assessed reversal of HMCESSRAP‐WT or ‐E127A‐DPCs in dsDNA at the indicated temperatures for the indicated amount of time before analysis by denaturing SDS–PAGE. Indeed, autocatalytic reversal was significantly delayed at 20°C.
-
HQuantification of DPC reversal assays using HMCESSRAP‐WT and ‐E127A shown in (G).
-
IThe extracts used in the replication reactions shown in (J and K) were immunoblotted for SPRTN, Rev1 and HMCES.
-
JAs an alternative additional strategy to determine whether reversal contributes to HMCES‐DPC resolution, we tested whether REV1 depletion results in stabilization of HMCES‐DPCs, reasoning that blocking TLS (and transfer of the DPC into dsDNA) may inhibit reversal. pICL‐lacO AP was replicated in mock‐, REV1‐, SPRTN‐ or REV1‐ and SPRTN‐depleted egg extracts, as indicated. At the indicated time points, plasmid was recovered under stringent conditions, the DNA was digested and released proteins were separated by SDS–PAGE. HMCES was detected by blotting. As expected, depletion of SPRTN alone resulted in a strong stabilization of HMCES‐DPCs. Depletion of REV1 alone did not stabilize HMCES‐DPCs, consistent with our data indicating that SPRTN represents the dominant mechanism for HMCES‐DPC resolution in egg extract. Surprisingly, when combined with SPRTN depletion, REV1 depletion partially suppressed the accumulation of HMCES‐DPCs. Superficially, this result is contrary to our expectations based on data presented in Fig 6. However, we interpret the result to indicate when the HMCES‐DPC is maintained at an ssDNA/dsDNA junction due to inefficient TLS, residual SPRTN or another protease can eventually degrade the HMCES‐DPC. Therefore, while these data do not provide evidence for HMCES‐DPC reversal during ICL repair in egg extract, they do reinforce the need for alternative removal mechanisms for HMCES‐DPCs present in dsDNA that are refractory to proteolysis.
-
KIn parallel with the reactions shown in (J), pICL‐lacO AP was replicated in the indicated egg extracts supplemented with [α‐32P]dCTP. Replication intermediates were separated on a native agarose gel and visualized by autoradiography. SC, supercoiled. OC, open circular. Consistent with a TLS defect upon Rev1 depletion, we observed accumulation of gapped, circular plasmids in replication gels, implying that the HMCES‐DPC is maintained at an ssDNA‐dsDNA junction.
Data information: Data in (H) represent the mean of three independent experiments ± SD.
