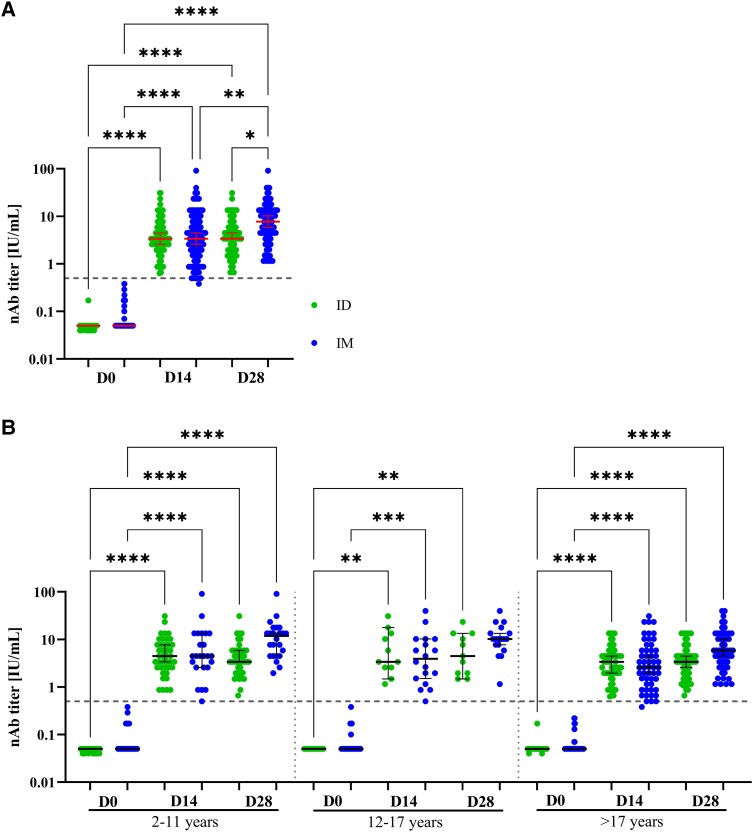
Figure 2.
Development nAb titers for intradermal (ID) and intramuscular (IM) vaccination: Titers of rabies neutralizing antibodies (nAb) measured by fluorescent antibody virus neutralization test (FAVNT) at baseline (D0), 14 d (D14) and 28 d (D28) after initial rabies vaccination either via ID (green) or IM administration (blue). A, Overall nAb development for full study cohort (n = 203, ID: 108, IM: 95). B, Results of nAb development stratified by age into children of 2–11 y (n = 65; ID: 41, IM: 24), teenagers of 12–17 y (n = 29; ID: 11, IM: 18), and adults of ≥17 y (n = 109; ID: 56, IM: 53). Each dot represents a single individual. Red lines represent median and interquartile range for each group. Dashed line indicates the threshold for seroprotection at 0.5 IU/mL. Statistics: Kruskal–Wallis with Dunn correction for multiple comparisons. *P < .05 **P ≤ .01, **P ≤ .001 and ****P ≤ .0001.