Abstract
Introduction and importance
Urothelial bladder cancer can infrequently result in cardiac metastasis, and be usually diagnosed in severe clinical conditions. We report a urothelial bladder cancer with cardiac metastasis and perform a literature review of published cases of transitional cell carcinoma (TCC) of the bladder with cardiac metastasis from 1934 to 2023 published in Pubmed.
Case presentation
42-year-old woman with urinnary bladder TCC, underwent radical cystectomy, developing cardiac metastasis after 25 months, cardiac surgery for partial removal of the lesion and using pembrolizumab with the highest reported survival to date.
Clinical discussion
After analysis of 20 case reports in the world among our case, men are more affected, tobacco exposure was the most prevalent risk factor, baseline T3 staging was the most common, and right ventricular and myocardium metastases are more prevalent. The most common symptoms were respiratory failure, changes in cardiac auscultation, and loss of weight. Six patients had cardiac tamponade, and the mean of drained fluid was 1040 ml. Immunohistochemical markers, such as CK7 and Calretinin, were decisive in elucidating the diagnosis. The average time between diagnosis of TCC and cardiac metastasis was 48.69 months, and the survival time after diagnosis of cardiac metastasis was 60.69 days.
Conclusion
Bladder TCC with cardiac metastasis is rare and with a low survival rate after the diagnosis. Patients with more advanced stages of TCC deserve diagnostic suspicion of cardiac metastasis if they progress with previously unreported respiratory and cardiac symptoms.
Keywords: Bladder cancer, Urothelial cancer (UC), Transitional cell carcinoma and cardiac cancer
Highlights
-
•
Urothelial bladder cancer with cardiac metastasis is rare.
-
•
We report a review of 20 papers published worldwide.
-
•
We present a case with the longest reported survival.
1. Introduction
Primary bladder cancer is the most common neoplasm of the urinary tract, and the histological type of transitional cell carcinoma (TCC) accounts for approximately 90 % of cases [1]. The most prevalent metastasis includes regional lymph nodes, the liver, lungs, and bones. Clinically diagnosed cardiac metastasis is quite rare and can be identified in approximately 10 % of cases of autopsies due to causa mortis of urothelial carcinoma [2,3].
2. Literature review
We performed a literature review of the main studies on TCC with cardiac metastasis with a search in the PubMed database (NCBI) between 1934 and 2023 with the following keywords: bladder cancer, urothelial carcinoma, transitional cell carcinoma and cardiac cancer, the Excluded works were those that were not open, were not in english language and did not have a minimum of information described as a case report. A total of 20 works were selected. Studies were included that described the epidemiological, clinical, surgical, histopathological and immunohistochemical characteristics, whose patients had bladder cancer (TCC) with metastasis to the heart, diagnosed in vivo or after autopsy (Table 1, Table 2, Table 3, Table 4).
Table 1.
Epidemiological analysis, diagnosis in life or autopsy, staging, diagnosis and previous treatment of patients diagnosed with transitional cell bladder carcinoma with metastasis to the heart.
Subtitle: W (Woman); M (Men); Age (years); Mass size in pathology* (cm); TUR bladder* (transurethral resection bladder); RT* (radiotherapy); CT* (chemotherapy); Surgical attachments* (total hysterectomy OR bilateral oophorectomy OR right nephrectomy OR prostate); New bladder reconstruction or urinary diversion* (Studer OR Briker surgery); CT adjuvant* (intravesical mitomycin).
Table 2.
Analysis of the consolidated data regarding the site of metastasis, affected cardiac layers, clinical symptoms and physical examination during diagnosis of metastasis, diagnostic tests, treatment used after diagnosis of metastasis.
Subtitle: RA* (right atrium); RV* (right ventricle); LV* (left ventricle); PA* (pulmonary artery); CNS* (central nervous system); AV* (aortic valve); LAG* (left adrenal gland); LNE* (lymph node enlargement inguinal, pelvic, retroperitoneal, periaortic abdominal); IVC* (inferior vena cava); Others* (bone, lymph nodes, kidneys and lungs); Asthenia* (Weakness, Fatigue, Increasing tiredness); Respiratory failure* (Tachypnea, Dyspnoea, Shortness of breath, Orthopnea, Severe hypoxemia); Cardiac auscultation changes (Heart murmur (holosystolic), irregular heart rhythm, muffled bubbling, pericardial friction, systolic murmur); LNE* (left inguinal lymph node enlargement); Edema* (lower extremity edema); ECG* (Eletrocardiograma- findings right heart axis deviation, incomplete right bundle branch block, atrial fibrillation); Chest X-ray* (showing increased cardiac silhouette, increased cardiac area, pleural effusion, heart failure); CT* (Computed tomography of the head or chest or abdomen); NMR* (nuclear magnetic resonance); PET-CT* (positron emission computed tomography); Tumor diameter* (Larger diameters of the metastasis mass evidenced by images in centimeters (cm)); Pericardiocentesis volume* (pericardiocentesis drained fluid volume (ml) or (cc/cm3)); Pericardial biopsy* (thoracic pericardiotomy or laparoscopyc); Cytology positive* (positive pericardial effusion metastasis); Heart mass biopsy* (ministernotomy, sternotomy or cardiac catheterization); Positive biopsy* (Heart mass biopsy positive for metastasis); RT* (Radiotherapy); Chemotherapy* (main drugs used: MVAC (methotrexate, vinblastine, doxorubicin and cisplatin), gemcitabine, carboplatin); Immunotherapy* (atezolizumabe,pembrolizumabe); Surgical* (sternotomy with partial resection of the cardiac mass in about 70 % of the total, reconstruction of the right atrium).
Table 3.
Analysis of the immunohistochemical pattern of biopsies or surgical specimen of cardiac metastasis.
PD-L1* (Programmed cell death 1 ligand 1); CK-5* (Cytokeratin 5); CK-7* (Cytokeratin 7); Ki-67* (Ki-67 Monoclonal Antibody or MIB-1); CK-20* (Cytokeratin 20); EMA* (Epithelial membrane antigen); AE1* cytokeratins; AE3 cytokeratins; MUC1 and MUC2* (Mucin 1 and Mucin 2); MOC-31* (MOC-31 antibody); p63* (p63 protein); CD 138* (CD138 antibody); HMWK* (High Molecular Weight Keratin); S-100* (Protein S100); PSA* (Prostate Specific Antigen); SMA* (Smooth Muscle Actin); CD34* (CD34 antibody); CD56* (CD56 antibody); CD45* (CD45 antibody); CD117* (CD117 antibody); TTF-1* (Thyroid Transcription Factor-1); HMB-45* (Human Melanoma Black); CDX2* (CDX2 antibody); HHF-35* (Muscle Specific Actin antibody); WT-1* (Wilms Tumor protein 1); PAX 8* (PAX 8 antibody); D2–40* (monoclonal antibody D2–40).
Table 4.
Analysis of the time between the diagnosis of bladder cancer and cardiac metastasis, whether the patient was alive or deceased at the end of the report and the time between the diagnosis of metastasis and death or follow-up time until the end of the report.
| Author | Year | No. of patients | Diagnosis of metastasis in life | Diagnosis metastasis autopsy postmortem examinations | Time between diagnosis of bladder cancer and diagnosis of cardiac metastasis (months) | Patient alive after end of report | Patient died after end of report | Time between diagnosis of metastasis until death or follow-up time until the end of reporting for patients who remained alive** (days) |
|---|---|---|---|---|---|---|---|---|
| Spooner et al. [5] | 1934 | 1 | + | AD* | NR | |||
| Fetter TR et al. [6] | 1959 | 3 | + | AD | NR | |||
| Babaian et al. [7] | 1980 | 8 | + | AD | NR | |||
| Gibbs et al. [8] | 1985 | 1 | + | 36 | + | 10** | ||
| Fabozzi et al. [9] | 1995 | 1 | + | 19 | + | 180 | ||
| Clemente et al. [2] | 1997 | 1 | + | 6 | + | FD | ||
| Kemp et al. [10] | 1997 | 1 | + | 4 | + | 1 (36 h) | ||
| Lin et al. [11] | 2000 | 1 | + | 60 | + | 30 | ||
| Malde et al. [12] | 2006 | 1 | + | 204 | + | 1 (5 h) | ||
| Teixeira et al. [13] | 2007 | 1 | + | AD | + | 14 | ||
| Mountzios et al. [14] | 2008 | 1 | + | 72 | + | 360 | ||
| Spiliotopoulos et al. [15] | 2008 | 1 | + | 60 | + | 360** | ||
| Peck et al. [16] | 2012 | 1 | + | 4 | + | 60 | ||
| Doshi et al. [17] | 2013 | 1 | + | 108 | + | 30** | ||
| Yamac et al. [18] | 2014 | 1 | + | AD | + | 14 | ||
| Selder et al. [19] | 2015 | 1 | + | AD | + | 1 | ||
| Khan et al. [20] | 2016 | 1 | + | NR* | + | FD | ||
| Yeaman et al. [21] | 2017 | 1 | + | NR | + | 6 | ||
| Palam et al. [22] | 2018 | 1 | + | 10 | + | NR** | ||
| Helal et al. [23] | 2022 | 1 | + | 12 | + | 120 | ||
| Our case | 2023 | 1 | + | 38 | + | 1403** |
AD* (Autopsy Diagnosis); NR* (Not Reported); FD (few days).
In our analysis, papers reporting clinical weakness, fatigue, and increasing tiredness were grouped under the complaint of ‘asthenia’; tachypnea, dyspnoea, shortness of breath, orthopnea, and severe hypoxemia were grouped into ‘respiratory failure’; changes such as heart murmur (holosystolic), irregular heart rhythm, muffled bubbling, pericardial friction, systolic murmur were grouped as ‘cardiac auscultation changes’; descriptions of right heart axis deviation, incomplete right bundle branch block, and atrial fibrillation were grouped into ‘electrocardiogram changes’; reports of showing an increased cardiac silhouette, increased cardiac area, pleural effusion, heart failure were grouped as ‘chest X-ray changes’. In Table 4, patients with a time frame between 5 h and 36 h from the diagnosis of metastasis to death were considered to have died equal to 1 day, for statistical analysis. Our work followed the recommendations and was reported according to the SCARE criteria [4].
3. Case report
A 42-year-old caucasian female, weight 51 kg (kg), height 1.55 m (m), body surface area 1.48 m2, body mass index 22.66 (kg/m2), performance scale type 1(ECOG), denies smoking, alcoholism and occupational exposures.
In February 2016, she sought a public health emergency service with recurrent urinary tract infection, hematuria, and pain in the lower abdomen. Laboratory tests showed hemoglobin of 10.6 mg/dl, urine test with hematuria and pyuria, normal renal function, and negative urine culture. Ultrasonography (US) of the kidneys and urinary tract showed expansive lesion covering the right bladder floor and the distal portion of the right ureter, measuring 4.0 × 2.3 × 3.0 cm (cm), right severe hydronephrosis, nephrolithiasis on the left in the lower calyx of 0.4 cm and middle calyx of 0.8 cm, ipsilateral megaureter (Fig. 1). Computed tomography (CT) of the abdomen evidenced an expansive lesion invading the right ureterovesical junction (UVJ), absence of ascites and lymph node enlargement, leading to right hydronephrosis, corresponding to a primary vesical neoplastic process and chest X-ray without signs of secondary implant.
Fig. 1.
Bladder US with expansive lesion on the right and distal portion of the right ureter, measuring 4.0 × 2.3 × 3.0 cm.
She underwent cystoscopy and transurethral resection (TUR), which showed a tumor lesion compromising the right lateral trigonal bar and right meatus, right lateral wall, and supratrigonal region, approximate size of 4 cm, sessile, macroscopically solid tumor. Anatomopathological (AP) confirmed a urothelial bladder carcinoma, pT2N0M0, high grade with involvement of the muscular layer. A Neoadjuvant 4 cycles chemotherapy with paclitaxel (80 mg/m2), cisplatin (70 mg/m2), and gemcitabine (1000 mg/m2) (PCG), and passage of double J on the right due to hydronephrosis was implemented.
Four months after the diagnosis, the patient was transferred to our service for a surgical approach and underwent a radical cystectomy with Studer's ileal neobladder, total hysterectomy, bilateral oophorectomy, lymphadenectomy of the obturator, iliac and extended pelvic chains, and right nephrectomy due to the kidney exclusion. It was an eight hour length surgery, and the hospital discharge was at the seventh day.
The cystectomy AP report showed a high-grade invasive urothelial carcinoma which was invading the bladder and right ureter, within free margins. 20 lymph nodes were also analyzed, and none compromised - pT3N0M0.
In the first month after surgery, she had some complications such as pyelonephritis with uroculture that showed multidrug-resistant Klebsiella pneumoniae with a regimen of amikacin-sensitive antibiotic therapy for 14 days; control exams showed a deterioration of renal function, an abdomen CT and USG detected hydronephrosis on the left due to an obstructive ureterolithiasis (0.9 cm middle third). We opted to start meropenem for 14 days and perform a left nephrostomy with the return of renal function and urinary output. In the control CT of the abdomen, there was a permanence of ureterolithiasis. Therefore, an ureterolithotripsy was indicated, and a stenosis of the neobladder UVJ was observed. After some clinical improvement, a new surgical approach was done for a left ureteral reimplantation in the neobladder, after a ureterolithotomy.
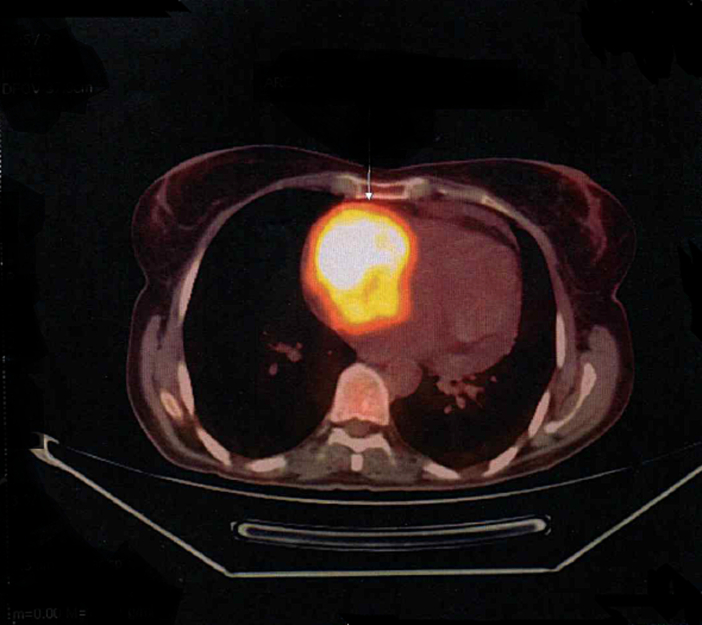
The patient remained in follow-up with Urology and Oncology teams without the need for new chemotherapy regimens. After 25 months of cystectomy and in follow-up, the patient reported weakness, fatigue, hyporexia, dizziness, intermittent fever and weight loss of 7 kg in 3 months, anemia of 9.2 mg/dl, physical examination without alterations. After requesting a CT scan of the chest and abdomen, she showed left nephrolithiasis of 3 mm with normal ureter caliber, and a small pericardial effusion. Due to the risk of tumor recurrence, laboratory investigation was initiated with iron kinetics, upper gastrointestinal endoscopy (UGE), colonoscopy, bone scintigraphy, and positron emission tomography (PET-FDG). The PET-FDG identified a lymph node of 1.4 cm, SUV of 13.9 in the obturator chain right, and an area of hypermetabolism in the right atrium (RA) (Fig. 2 and Supplementary Fig. 1).
Fig. 2.
PET-FDG with hypermetabolism area in the right atrium in axial view.
Supplementary Fig. 1.

PET-FDG with area of hypermetabolism in the right atrium in coronal section.
The patient was referred to the cardiac surgery team, a transthoracic echocardiogram (TTE) and magnetic resonance imaging of the heart (MRI) were requested, TTE showed hyperechogenic 4.7 × 4.1 cm mass in RA, covering the entire atrial cavity, suggesting adherence in the interatrial septum, moderate pericardial effusion, MRI confirmed that it was a probable expansive neoplastic lesion in the RA, encompassing the interatrial septum, lateral wall and roof of the RA, lateral wall of the right ventricle (RV), and right lateral wall of the ascending aorta measuring about 7.0 × 7.0 × 6.8 cm, with moderate pericardial effusion, without pericardial involvement (Supplementary Fig. 2, Supplementary Fig. 3). An RA biopsy was performed with immunohistochemistry (IHC) showing positivity for AE1 and AE3, CK7, ki-67 (MIB1), negativity for smooth muscle actin, myogenin, S-100, CD 45, Gata 3, CK 20, calretinin and nonspecific for p63 protein, conclusion with findings of atypical epithelioid cells (Supplementary Fig. 4, Supplementary Fig. 5, Supplementary Fig. 6, Supplementary Fig. 7, Supplementary Fig. 8). Twenty-nine months after the radical cystectomy, the decision was made to resect the cardiac metastasis and underwent sternotomy with median pericardiotomy and cardiopulmonary bypass with evidence of a large hardened tumor mass invading the RA wall, inter-atrial septum, and towards the tricuspid valve and RV was identified (Supplementary Fig. 9). Partial resection of the mass was carried out in about 70 % of the total, repair of the inter-atrial communication and repair of the tricuspid valve, reconstruction of the RA with bovine pericardium. The AP report of the cardiac surgery showed a mass measuring 7.0 × 6.0 × 2.5 cm and a diagnosis of poorly differentiated infiltrating carcinoma in the RA wall, IHC with positivity for programmed cell death 1 ligand (PD -L1) with CPS (combined positive score) >10 %, CK5, CK7, p63, GATA3 and negative for RE, CK20, chromogranin A, synaptophysin, CDX-2, TTF-1, PAX-8, concluding a metastatic urothelial carcinoma (Supplementary Fig. 10, Supplementary Fig. 11, Supplementary Fig. 12, Supplementary Fig. 13, Supplementary Fig. 14).
Supplementary Fig. 2.

TTE showing a 4.7 × 4.1 cm hyperechogenic mass in the right atrium.
Supplementary Fig. 3.
MRI of the heart with expansive lesion in the right atrium encompassing the interatrial septum, lateral wall and roof of the right atrium, lateral wall of the right ventricle.
Supplementary Fig. 4.
HE 100× - Endomyocardial biopsy - panoramic view of areas showing hyperbasophilia with signs of compression, possibly corresponding to increased cellularity and nuclear hyperconcentration.
Supplementary Fig. 5.
IHC 100× - AE1/AE3, PanCK - positive in foci - probable epithelial cells.
Supplementary Fig. 6.
IHC - AE1/AE3, PanCK - 400× positive in atypical epithelial cells, favoring epithelial origin.
Supplementary Fig. 7.
IHC - CK7 - positive in epithelial cells.
Supplementary Fig. 8.
IHC - Ki67 - positive in a subpopulation of epithelial cells, showing proliferative activity.
Supplementary Fig. 9.

Surgical procedure with partial resection of the mass and repair of the tricuspid valve with bovine pericardium.
Supplementary Fig. 10.

IHC - CK5.
Supplementary Fig. 11.

IHC - P63.
Supplementary Fig. 12.

IHC - GATA3.
Supplementary Fig. 13.

IHC – TC.
Supplementary Fig. 14.

PD-L1 22C3 primary antibody exhibiting weak to strong linear membrane staining of tumor cells (20×). In this case, the Combined Positive Score (CPS) was >10.
Thirty months after the cystectomy and after removal of 70 % of the total mass, due to the residual tumor mass in the RA, an immunohistochemical analysis for PD-L1 was requested, showing positivity (CPS > 10 %), palliative treatment was started since december 2019 with pembrolizumab (PD-L1 inhibitor), currently using 100 mg/4 ml (400 mg) of pembrolizumab every 40 days and clinical follow-up with annual PET-FDG- showing current resolution of hypermetabolism in the cardiac area with tumor stabilization (Fig. 3). After 73 months of radical cystectomy, the patient continues with oncological and urological follow-up, undergoing annual PET-FDG without evidence of hypermetabolic lesions, use of immunotherapy with pembrolizumab and intermittent bladder catheterization.
Fig. 3.
PET-FDG showing resolution of hypermetabolism in the heart area.
4. Discussion
The analysis of 20 studies, plus our case report totaled 30 patients described between 1934 and 2023, proving the rarity of cardiac metastasis of TCC [2,[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]. Data in showed that patients with cardiac metastasis from TCC are predominantly men (77.78 %), mean age 65.47 years, most patients were diagnosed after death by autopsy (57.90 %) (Table 1 and Fig. 4). Caucasian ethnicity, and comorbidities such as exposure to smoking and arterial hypertension were more prevalent. Only 10 studies [2,[9], [10], [11], [12],[14], [15], [16],18,23] report the TNM staging profile of TCC bladder cancer, with tumors T1 (20.00 %), T2 (10.00 %), T3 (50.00 %), T4 (20.00 %), WHO classification in grade 2 (12.50 %), grade 3 (75.00 %), grade 4 (12.50 %). The size of the primary bladder mass ranged from 2.0 cm to 7.3 cm and a mean of 4.32 cm. Most diagnoses occurred with the aid of cystoscopy and transurethral resection. 18 studies [2,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]]reported some type of treatment administered after a diagnosis of TCC, most patients were treated with radical cystectomy (50.00 %), chemotherapy (neoadjuvant or adjuvant) (44.45 %), and bladder reconstruction or urinary diversion (33.34 %) (Table 1).
Fig. 4.
Histogram analyzing gender, age and frequency of all cases published in the world.
The evaluation of the 16 studies [2,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19],21,23] that reported the site of cardiac metastasis and other concomitant organs showed that the right ventricle (68.75 %) was the most affected, and with right atrium involvement and regional or distant lymph node enlargement (31.25 %) also being prevalente (Table 2).
The myocardium (64.28 %) is the most affected layer, followed by the pericardium and endocardium (42.85 %). The clinical complaints and changes in the physical examination most reported before the diagnosis of metastasis are respiratory failure (43.34 %), changes in cardiac auscultation (26.67 %), weight loss (26.67 %), tachycardia (23.34 %), asthenia (20.00 %) and hematuria (16.67 %). The most used exams for the diagnosis of cardiac metastasis were transthoracic echocardiogram (53.34 %), computed tomography (46.67 %), chest X-ray (23.34 %), electrocardiogram (16.67 %), and the diameter of the metastasis ranged from 1.3 to 7.0 cm, mean 5.20 cm. Twelve papers [8,9,[14], [15], [16],[18], [19], [20], [21], [22], [23]] reported pericardial effusion, and 6 papers [8,9,15,[20], [21], [22]] reported cardiac tamponade, and 8 papers [8,9,[14], [15], [16],[20], [21], [22]] described diagnostic pericardiocentesis. The drained volume ranged from 700 to 2000 ml, mean of 1040 ml; 6 studies [8,9,14,[20], [21], [22]] had information on positive oncotic cytology, and of the 6 cardiac biopsies [10,11,[14], [15], [16]] reported, 4 biopsies [11,[14], [15], [16]] (83.34 %) were confirmed as cardiac metastasis. Twelve papers [8,9,11,[14], [15], [16], [17],[20], [21], [22], [23]] reported the diagnosis of cardiac metastasis in vivo and only 7 papers [9,14,15,17,22,23] were able to implement some treatment after the diagnosis of metastasis, 6 papers [9,14,15,17,22] reported complementary treatment with chemotherapy (85.71 %), followed by immunotherapy (42.85 %), surgery, and radiotherapy (28.57 %) (Table 2).
Only 7 studies [11,[14], [15], [16],20,22] used immunohistochemical markers for diagnostic elucidation of the biopsy of cardiac metastasis, 5 of which were cardiac biopsies [10,11,[14], [15], [16]] and 4 pericardial biopsies [8,9,20,22], and our report with analysis of the surgical specimen (Table 3). When analyzing the samples, 17 markers were positive: Keratin (1/100 %), PD-L1 (1/100 %), CK7 (5/100 %), Ki-67 (1/100 %), EMA (2/ 100 %), AE1 (3/100 %), AE3 (2/100 %), MUC1 and MUC2 (2/100 %), MOC-31 (1/100 %), Vimentin (1/100 %), p63 (2/100 %), Pancytokeratin (1/100 %), CD138 (1/100 %), HMWK (1/100 %), Uroplakin (2/4 50.00 %) and CK-20 (1/4 25, 00 %); 26 markers were negative Leukocyte common antigen (1/100 %), CK20 (3/4 75.00 %), S-100 (3/100 %), PSA (1/100 %), Uroplakin (2/4 50, 00 %), SMA (2/100 %), Actin (1/100 %), CD34 (2/100 %), CD56 (1/100 %), CD45 (2/100 %), CD117 (2/100 %), Synaptophysin (2/100 %), Chromogranin (2/100 %), Myogenic differentiation transcription factor 1 (1/100 %), TTF-1 (3/100 %), Melan-A (1/100 %), HMB-45 (1/100 %), Myogenin (2/100 %), Desmin (2/100 %), CDX2 (2/100 %), Thrombomodulin (2/100 %), HHF-35 (1/100 %), WT-1 (1/100 %), PAX 8 (1/100 %), D2–40 (1/100 %), Calretinin (5/100 %) (Table 3).
Thirteen studies [2,[8], [9], [10], [11], [12],[14], [15], [16], [17],22,23] evaluated the time between the diagnosis of bladder cancer and cardiac metastasis, which ranged from 4 months to 204 months, mean of 48.69 months (Table 4). Only 5 papers [8,15,17,22] reported that the patients survived until the end of the case report after the diagnosis of cardiac metastasis, ranging from 10 to 1403 days of survival, a mean of 450,75 days considering only 4 studies with time report in days. Thirteen studies [2,[9], [10], [11], [12], [13], [14],16,[18], [19], [20], [21],23] reported the time between the diagnosis of cardiac metastasis and reported death, which ranged from 5 h to 360 days, mean of 60.69 days of life, considering reports of hours and a short time such as at least one day (Table 4).
Cancers with cardiac metastasis are quite rare, and the latest reviews report an incidence of 2.3 to 18.3 % [24]. In our review, most diagnoses occurred in deceased or autopsy, with male prevalence and a mean age of 65.47 years. Exposure to smoking and high blood pressure were the most prevalent risk factors, most patients had more advanced staging T3. Literature data have already shown that metastases occur predominantly in the presence of local tumor invasion and intense lymphatic spread. In our review, the most described locations of the cardiac implant were the right ventricle, myocardium and pericardium. Other reviews determined that the pericardium is involved in about two-thirds of all cardiac metastases [22,24]. Pericardial effusion is an expected progression of the disease, and the severe clinical form of cardiac tamponade occurred in 6 studies [8,9,15,[20], [21], [22]] and some authors estimate that about 60 ml of pericardial fluid is needed to ensure a good analysis of pericardial cytology, and the combination of cytology with pericardial biopsy increases the overall sensitivity for the diagnosis of malignancy by 8 % [25,26,27].
The diagnosis of cardiac metastasis has important limitations due to the complex surgical approach and the patient's clinical condition, which may preclude the biopsy. Regarding immunohistochemistry markers, 17 markers were positive; the most used were CK7 and AE1, with positivity in 100 % of cases, CK-20 showed 25 % positive and 75 % negative and Uroplakin showed 50 % positive and 50 % negative, showing low specificity in diagnostic elucidation, Calretinin was used 5 times and all were negative.
The rate of survival of patients with cardiac metastasis from TCC is extremely low, as most diagnoses occur in a severe clinical state with the impossibility of effective treatment. TCC prognosis depends on the stage of disease, with 5-year survival for stage 1 TCC of up to 85 % compared with 15 % for stage 4 disease [28]. Hattori et al. in their review of 14 cases of urothelial metastasis to the pericardium report that most patients died within one year after the diagnosis of symptoms of cardiac metastases [29]. Our review found that the time between the diagnosis of cardiac metastasis and the reported death ranged from 5 h to 360 days with a mean of 60.69 days.
PD-L1 and its ligands are expressed in UC and some studies have shown that inhibition of the PD-L1 pathway has clinical benefit in patients with locally advanced or metastatic UC. Plimack et al. showed that pembrolizumab showed antitumor activity and acceptable safety in patients with advanced urothelial disease [30]. Pembrolizumab was shown in a phase III study to be associated with improved survival and quality of life compared to chemotherapy [31]. The use of pembrolizumab appears well defined in patients with locally advanced or metastatic UC who are not eligible for cisplatin-containing therapy and whose tumors express PD-L1 (CPS ≥ 10 %) [31].
5. Conclusion
Cardiac metastasis from TCC is a rare event, most patients are diagnosed with cardiac metastasis in advanced stages with severe clinical symptoms. This review aims to show relevant data from 20 articles on cardiac metastasis of TCC and its outcomes, for better elucidation of the disease and management of new cases.
The following are the supplementary data related to this article.
Financing source
None.
Declaration of competing interest
None.
Acknowledgments
We would like to thank the Urology Oncology Service of the University of Brasília Hospital (HUB/UnB) for their help and assistance during the elaboration of this article.
References
- 1.Ploeg M., Aben K.K.H., Kiemeney L.A. The present and future burden of urinary bladder cancer in the world. World J. Urol. 2009;27(3):289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemente L.M., Patier J.L., Lopez-Suanzes M.J., Jimenez M. Cardiac metastases from a transitional cell carcinoma: an unusual clinical manifestation. Br. J. Urol. 1997;80(5):831–832. doi: 10.1046/j.1464-410x.1997.00394.x. (PMID: 9393319) [DOI] [PubMed] [Google Scholar]
- 3.Klatt E.C., Heitz D.R. Cardiac metastases. Cancer. 1990;65(6):1456–1459. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Agha R.A., Franchi T., Sohrab C., Mathew G., Kirwan A., Thomas A., et al. The SCARE 2020 guideline: updating consensus surgical case report (SCARE) guidelines. Int. J. Surg. 2020;84(1):226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Spooner A.D. Metastasis in epithelioma of urinary bladder. Tr. Am. Assoc. Genito-Urin. Surg. 1934;27:81. [Google Scholar]
- 6.Fetter T.R., Bogaev J.H., McCuskey B., Seres J.L. Carcinoma of the bladder; sites of metastases. J. Urol. 1959;81(6):746–748. doi: 10.1016/s0022-5347(17)66104-4. (PMID: 13655397) [DOI] [PubMed] [Google Scholar]
- 7.Babaian R.J., Johnson D.E., Llamas L., Ayala A.G. Metastases from transitional cell carcinoma of urinary bladder. Urology. 1980;16(2):142–144. doi: 10.1016/0090-4295(80)90067-9. (PMID: 7404907) [DOI] [PubMed] [Google Scholar]
- 8.Gibbs J.L., Rao R.S., Williams G.J. Polypoid tumour of the pericardium--a previously unrecognised macroscopic appearance of metastatic bladder carcinoma. Int. J. Cardiol. 1985;8(2):205–208. doi: 10.1016/0167-5273(85)90288-8. (PMID: 4008108) [DOI] [PubMed] [Google Scholar]
- 9.Fabozzi S.J., Newton J.R., Jr., Moriarty R.P., Schellhammer P.F. Malignant pericardial effusion as initial solitary site of metastasis from transitional cell carcinoma of the bladder. Urology. 1995;45(2):320–322. doi: 10.1016/0090-4295(95)80025-5. (PMID: 7855982) [DOI] [PubMed] [Google Scholar]
- 10.Kemp W., Rothberg M., Saporito J.J., et al. Transitional cell carcinoma and right ventricular obstruction. J. Urol. 1997;158:1522–1523. http://dx.doi.org/10.1016/S0022-5347(01)64260-5. [PubMed] [Google Scholar]
- 11.Lin W.C., Telen M.J. Cardiac metastasis from a transitional cell carcinoma: a case report. Med. Oncol. 2000;17(2):147–150. doi: 10.1007/BF02796211. (PMID: 10871822) [DOI] [PubMed] [Google Scholar]
- 12.Malde D.J., Gall Z., George N. Ventricular rupture secondary to cardiac metastasis of transitional cell carcinoma of the bladder. Scand. J. Urol. Nephrol. 2006;40(2):170–171. doi: 10.1080/00365590510040372. (PMID: 16608819) [DOI] [PubMed] [Google Scholar]
- 13.Teixeira M.A.B., Venchiarutti Camila, Motta; Teixeira, Ralcyon Francis Azevedo; Sacilotto, Luciana; Teixeira, Carlos Osvaldo. Transitional cell carcinoma with metastasis to the aortic valve: case report with necropsy. Rev. ciênc. méd., (Campinas) 2007;16(4/6):279–285. jun.-dez. [Google Scholar]
- 14.Mountzios G., Bamias A., Dalianis A., Danias P., Pantelidaki E., Nanas J., Dimopoulos M.A. Endocardial metastases as the only site of relapse in a patient with bladder carcinoma: a case report and review of the literature. Int. J. Cardiol. 2010;140(1):e4–e7. doi: 10.1016/j.ijcard.2008.11.012. Epub 2008 Nov 30. PMID: 19046610. [DOI] [PubMed] [Google Scholar]
- 15.Spiliotopoulos K., Argiriou M., Argyrakos T., et al. Solitary metastasis of urothelial carcinoma of the urinary bladder to the heart: an unusual clinical manifestation. J. Thorac. Cardiovasc. Surg. 2008;136:1377–1378. doi: 10.1016/j.jtcvs.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Peck J.R., Hitchcock C.L., Maguire S., Dickerson J., Bush C. Isolated cardiac metastasis from plasmacytoid urothelial carcinoma of the bladder. Exp Hematol Oncol. 2012;1(1):16. doi: 10.1186/2162-3619-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doshi T.V., Doshi J.V., Makaryus J.N., Makaryus A.N. A rare case of successfully treated cardiac metastasis from transitional cell bladder cancer. Am. J. Ther. 2013;20(3):307–310. doi: 10.1097/MJT.0b013e3181f6c131. [DOI] [PubMed] [Google Scholar]
- 18.Yamac A.H., Insanic D., Bockmeyer C. Cardiac metastasis from a urothelial cell carcinoma: a commented case report. Cardiovasc. Pathol. 2014 May-Jun;23(3):178–180. doi: 10.1016/j.carpath.2014.01.005. 24560085 Epub 2014 Jan 17. [DOI] [PubMed] [Google Scholar]
- 19.Selder J.L., van Dekken H., de Voogt W.G. The heart is not too noble to host a metastatic tumor! A right ventricle metastasis of a transitional cell carcinoma of the bladder. Neth Heart J. 2015;23(1):74–76. doi: 10.1007/s12471-011-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan R., Jehangir W., Tulpule S., Osman M., Singh S., Sen S. Pericardial metastasis induced tamponade from urothelial carcinoma: a rare entity. Case Rep. Med. 2016;2016:6162732. doi: 10.1155/2016/6162732. (Epub 2016 Apr 11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman C.T., Patel M., Chen I. Rare presentation of urothelial bladder carcinoma as cardiac tamponade. Urol Case Rep. 2017;12(15):33–35. doi: 10.1016/j.eucr.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palam S., Kapoor R., Kulinski J. A rare presentation of cardiac tamponade from metastatic urothelial carcinoma of the bladder. Case Rep Cardiol. 2018;19(2018):6750264. doi: 10.1155/2018/6750264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helal C., Lambert T., Joly B.S., Dumont C., Gauthier H., Culine S. Disseminated intravascular coagulation with enhanced fibrinolysis caused by heart metastasis of bladder urothelial carcinoma. Case Rep Oncol. 2022;15(2):745–749. doi: 10.1159/000523822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J. Clin. Pathol. 2007;60(1):27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Islam N., Ahmedani M.Y. Renal carcinoma presenting as cardiac tamponade: a case report and review of literature. Int. J. Cardiol. 1998;64(2):207–211. doi: 10.1016/s0167-5273(98)00015-1. [DOI] [PubMed] [Google Scholar]
- 26.Rooper L.M., Ali S.Z., Olson M.T. A minimum volume of more than 60 ml is necessary for adequate cytologic diagnosis of malignant pericardial effusions. Am. J. Clin. Pathol. 2016;145(1):101–106. doi: 10.1093/ajcp/aqv021. [DOI] [PubMed] [Google Scholar]
- 27.Saab J., Hoda R.S., Narula N., et al. Diagnostic yield of cytopathology in evaluating pericardial effusions: clinicopathologic analysis of 419 specimens. Cancer Cytopathology. 2017;125(2):128–137. doi: 10.1002/cncy.21790. [DOI] [PubMed] [Google Scholar]
- 28.National Collaborating Centre for Cancer (UK). Bladder Cancer: Diagnosis and Management. London: National Institute for Health and Care Excellence (UK); 2015 Feb (NICE Guideline, No. 2.) 3, Diagnosing and staging bladder cancer.
- 29.Hattori S., Miyajima A., Maeda T., et al. Metastatic urothelial carcinoma to pericardia manifested by dyspnea from cardiac tamponade during systemic chemotherapy: case report and literature review. Canadian Urological Association Journal. 2012;6(5):E184–E188. doi: 10.5489/cuaj.11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plimack E.R., Bellmunt J., Gupta S., Berger R., Chow L.Q., Juco J., Lunceford J., Saraf S., Perini R.F., O’Donnell P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212–220. doi: 10.1016/S1470-2045(17)30007-4. (Epub 2017 Jan 10) [DOI] [PubMed] [Google Scholar]
- 31.Nadal R., Bellmunt J. Management of metastatic bladder cancer. Cancer Treat. Rev. 2019;76:10–21. doi: 10.1016/j.ctrv.2019.04.002. Epub 2019 Apr 15. PMID: 31030123. [DOI] [PubMed] [Google Scholar]