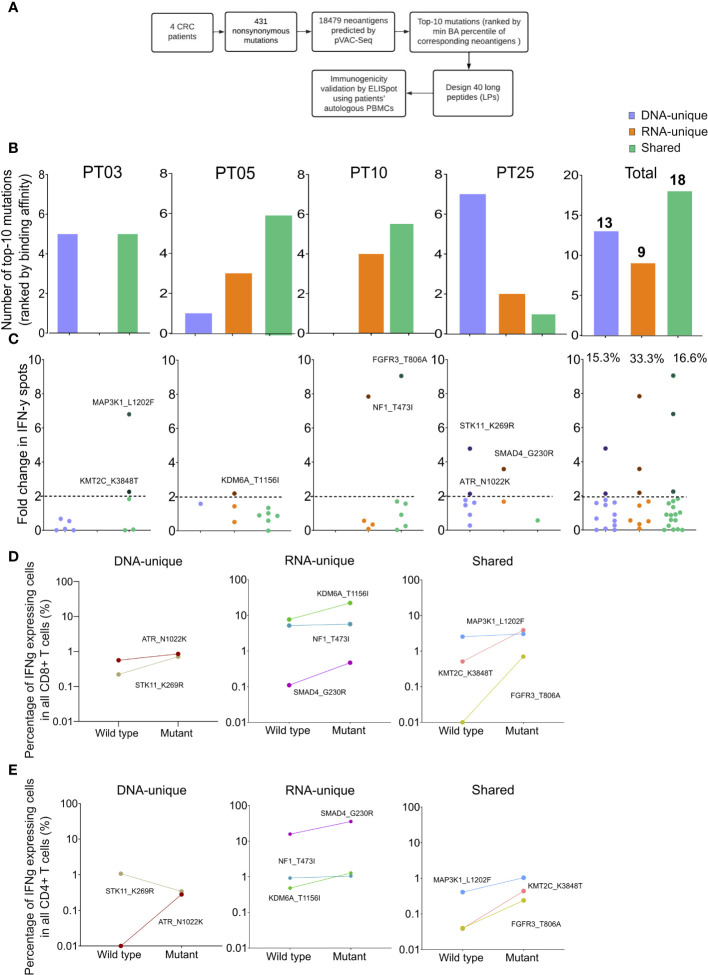
Figure 5.
Validation of neoantigens in silico identified from the modified workflow by ELISpot assays on four CRC patients. (A) A schematic diagram illustrates the procedural steps of neoantigen prioritization and the ELISpot assay. (B) The number of each type of neoantigens identified from each CRC patient. (C) The fold change in IFN-γ spots, relative to the wildtype peptides, for 40 long peptides. Note: only the mutants that result in a positive value in ELISpot are depicted with their corresponding amino acid change. (D) The percentage of IFN-γ expressing CD4+ T cells induced by indicated long peptides. Note: these long peptides induce a more than 2-fold change in IFN-γ spots as observed in the ELISpot assay. (E) The percentage of IFN-γ expressing CD8+ T cells induced by indicated long peptides.