Figure 4.
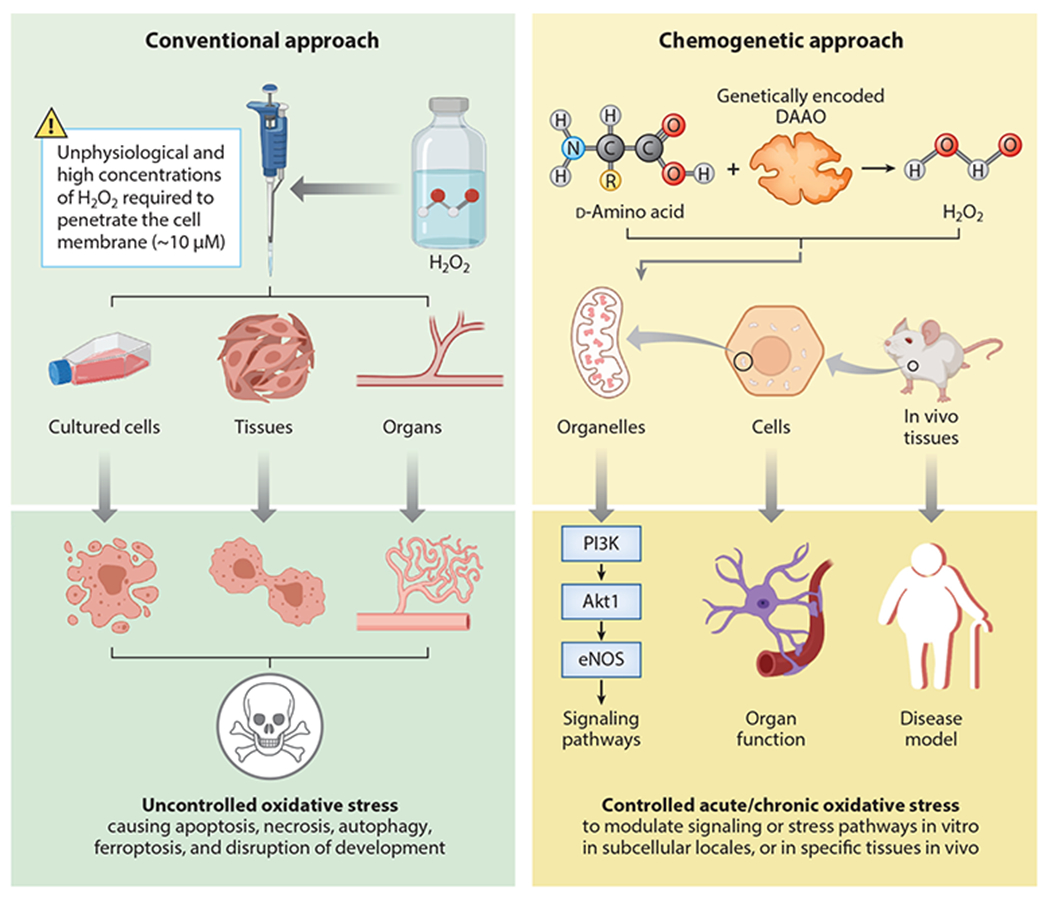
Comparison of conventional versus chemogenetic approaches to perturb cellular redox balance. (Left) Conventional approach to perturb intracellular redox balance by adding exogenous hydrogen peroxide (H2O2) or redox-active small molecules to cells or tissues. The direct addition of H2O2 to cells has highly variable effects on intracellular pathways due to the variable permeability of cell membranes to H2O2 and the differential distribution of H2O2 between intracellular organelles. Moreover, the concentrations of exogenous H2O2 needed to activate physiological pathways can also produce toxic effects on cells. Redox-active small molecules can also be added to cells, with variable (and often nonspecific) effects on intracellular oxidants. (Right) A chemogenetic approach that permits dynamic regulation of H2O2 generated by recombinant yeast d-amino acid oxidase (DAAO) expressed in mammalian cells. H2O2 can be reversibly generated in specific intracellular organelles by providing (or withholding) d-amino acids. DAAO can be cloned as a fusion protein along with the H2O2 biosensor HyPer to document H2O2 formation within specific subcellular compartments. Alternatively, the DAAO and HyPer can be differentially targeted to distinct subcellular organelles to study intracellular H2O2 diffusion from the site of H2O2 synthesis by DAAO to the organelle in which H2O2 is detected by HyPer. Chemogenetic approaches have been used both in cultured cells and in animals in vivo to dissect both physiological and pathophysiological oxidant-modulated pathways. Chemogenetic methods have enabled experimenters to isolate redox state as an independent variable of both in vitro and in vivo systems. Moreover, biosensors can be targeted to compartments distinct from the organelle expressing DAAO, enabling the experimenter to study the effects of oxidants generated in different subcellular locales. Figure adapted from images created with BioRender.com.
