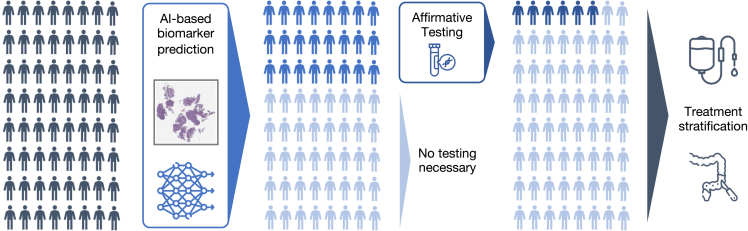
Figure 5.
Envisioned clinical workflow for the proposed MSI-high classifier on biopsies
This assumes the system reaches a sufficient performance in additional external validation and is approved as a medical device. This workflow would only apply to non-metastatic disease. Neoadjuvant immunotherapy is not yet recommended by medical guidelines but is backed up by Phase-II clinical trials. Not shown: tissue preprocessing and scanning pipelines and confirmatory tests of MSI-high after a positive deep learning-based pre-screening.