Figure 4. Mis-erasing of chromatin modification is critically involved in cancer initiation and progression.
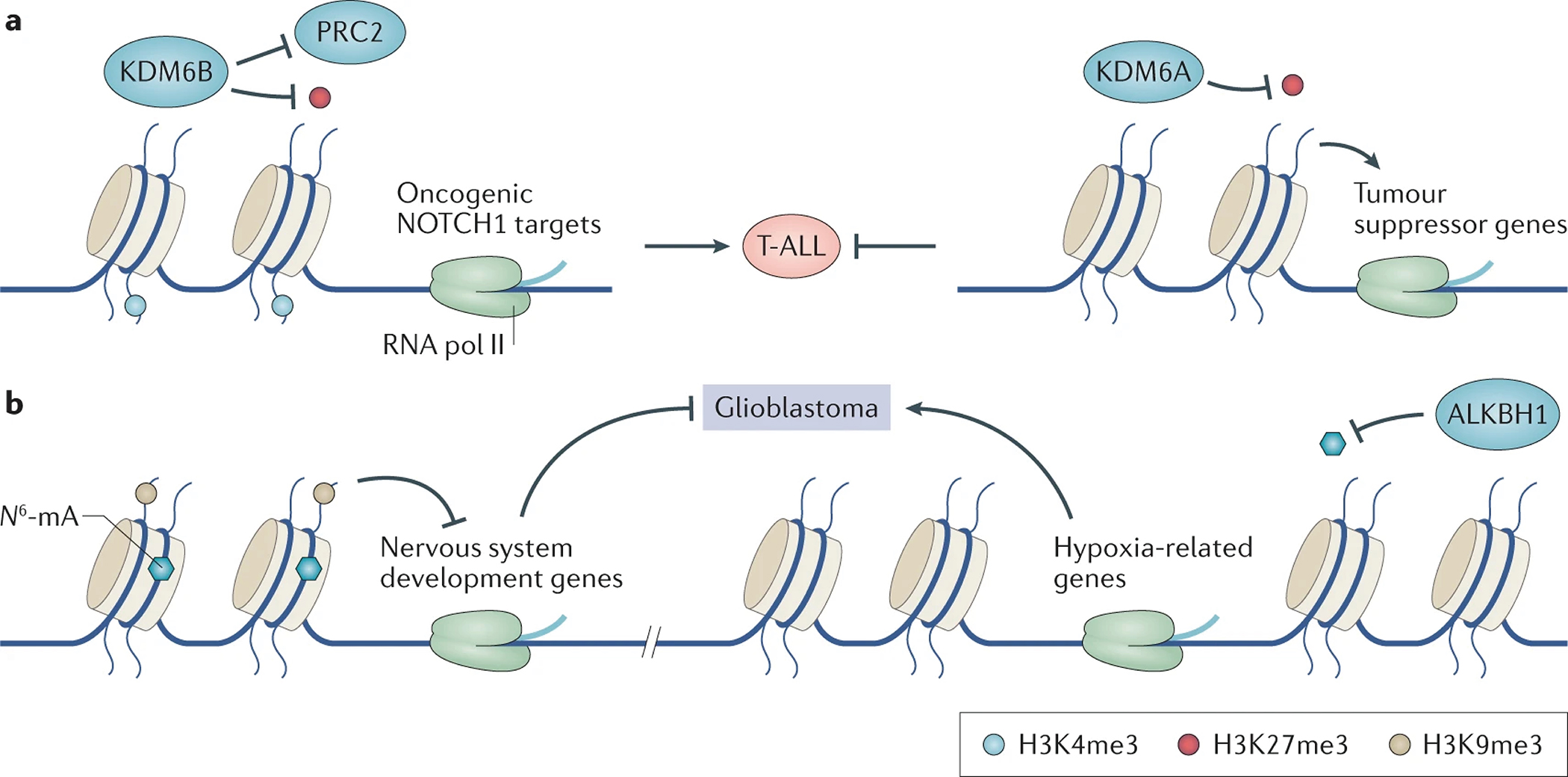
a. In T-ALL, the expression level of KDM6B increases while the (KDM6A level decreases. Both KDM6A and KDM6B are erasers of H3K27me3. KDM6B binds to oncogenic NOTCH1 target genes, catalyzes the demethylation of H3K27me3 and antagonizes the polycomb repressor complex 2 (PRC2), an H3K27me3 writer. Decreased H3K27me3 and increased H3K4me3 facilitate the expression of oncogenic genes. In contrast, KDM6A binds to tumor suppressor genes and facilitates their expression. KDM6A thus functions as tumor suppressor in T-ALL.
b. In glioblastoma stem cells (GSC), DNA adenine methylation (N6-mA) co-exists with H3K9me3, suppressing the neuronal differentiation-related gene-expression program. Depletion of nucleic acid dioxygenase ALKBH1 in glioblastoma facilitates silencing of oncogenic genes and thus decreases GSC proliferation.
