Abstract
Atopic dermatitis is a chronic inflammatory skin disorder associated with a heterogeneous presentation and considerable disease burden. Exploring atopic dermatitis treatment patterns and patient benefits could improve disease management and patients’ quality of life. This study aimed to describe current and previous atopic dermatitis treatment patterns and patient benefits from those treatments to inform disease management. Data were collected in 10 countries. Adults (n = 1,988) with confirmed moderate-to-severe atopic dermatitis completed a web-based cross-sectional survey. Most patients (86.6%) had body surface area involvement <10%, and therapies used were topical (69.7%), systemic (28.1%), and biologics (2.3%). Most flares were managed by topical monotherapies (73.4%), even in patients with body surface area involvement ≥10%. Treatment expectations were met only partially, or not at all, in 75% of patients. Those with body surface area involvement ≥10% reported lower treatment satisfaction. Overall, this study highlights the unmet medical needs in atopic dermatitis management.
SIGNIFICANCE
Atopic dermatitis is a skin disorder with considerable symptoms that can negatively affect a person’s quality of life. In this study, 1,988 patients with atopic dermatitis across 10 countries completed a survey to better understand the treatments they were taking, and how they were helping them. Most participants had mild skin symptoms and were using topical remedies only. Notably, three out of four patients said they were not satisfied with their treatments. This number increased in those with more severe symptoms. This study found that the needs of patients with atopic dermatitis are not being met by current treatments.
Keywords: atopic dermatitis, patient satisfaction, clinical practice patterns, disease management, patient outcome assessment
Atopic dermatitis (AD) is a chronic inflammatory skin disorder characterized by eczematous, highly pruritic, and often painful lesions, impacting patients’ sleep, well-being, and quality of life (QoL) (1). Moderate to-severe AD is defined as an Eczema Area and Severity Index (EASI) score ≥ 16 or < 16 plus ≥ 1 of the following conditions: localization to face, hands, or genitals; itch with a numerical rating score (NRS) > 7; sleep disturbances with NRS > 7; QoL impairment with Dermatology Life Quality Index (DLQI) >10 (2), or a minimum involvement of ≥ 10% body surface area (BSA), and usually warrants systemic therapy (2–5). Exploring AD treatment patterns and associated patient benefits is of importance when assessing disease severity (5) and assists in improving disease management (6) and enhancing patient QoL (7).
This study presents results from the Atopic Dermatitis Patient Satisfaction and Unmet Need Survey, which aimed to describe current and previous AD treatments and patient benefits with AD therapies in the real-world setting.
MATERIALS AND METHODS
This study was conducted in 10 countries (Australia (n = 201), Belgium (n = 194), Canada (n = 250), France (n = 250), Germany (n = 250), Italy (n = 252), Japan (n = 252), the Netherlands (n = 165), Spain (n = 250), and the UK (n = 250)) according to market research codes of conduct. Consenting adult participants were recruited via internet panels between July and September 2019 to complete a web-based, cross-sectional survey. The survey included adult patients (≥ 18 years (≥ 20 in Japan) to ≤ 75 years) with self-reported dermatologist-confirmed AD (or immunologist-confirmed AD in Australia), and excluded those with patient-rated mild AD and affected BSA < 3%.
The current analysis included adult participants who, at the time of the survey, were using topical monotherapies (topical corticosteroids, calcineurin inhibitors), or conventional systemic (cSys; systemic corticosteroids, immunosuppressants), or biologic AD treatments (dupilumab only at that time) (with or without topicals), and excluded those participants currently receiving phototherapy or combination therapy with cSys and biologics. Data collected included demographics, disease characteristics including patient-rated worst BSA in the last year (measured by the number of hand palms needed to cover the affected areas), treatment patterns (previous/current treatments, reasons for discontinuation, flare management), and treatment benefits (AD treatment expectations, the extent to which expectations were met). Outcomes were based on the Patient Benefit Index (8). Participants were stratified according to worst BSA involvement (BSA < 10% (mild) and BSA ≥ 10% (moderate/severe)). Data were analyzed descriptively.
RESULTS
A total of 1,988 adult patients were included in the analysis. Most patients (86.6%) had BSA < 10%, and mean disease duration was higher for patients with BSA ≥10% vs. BSA <10% (Table I).
Table I.
Baseline patient and treatment characteristics
| All n = 1,988 (100) | BSA <10% n = 1,721 (86.6) | BSA ≥10% n = 267 (13.4) | |
|---|---|---|---|
| Demographic data | |||
| Age, years, mean (SD) | 39.5 (12.9) | 39.3 (12.8) | 40.8 (13.3) |
| Male, n (%) | 678 (34.1) | 587 (34.1) | 91 (34.1) |
| Diagnosis age ≤18 years, n (%) | 1,019 (51.3) | 864 (50.2) | 155 (58.1) |
| Disease duration, years, mean (SD) | 19.5 (14.2) | 19.1 (13.9) | 22.5 (15.8) |
| Flare frequency over past year | |||
| Patients with ≥1 flare in the past year, n (%) | 1,804 (94.8) | 1,576 (94.9) | 228 (94.2) |
| Mean number of flares (SD) | 7.5 (20.1) | 6.8 (17.2) | 12.3 (33.4) |
| Median number of flares (IQR) | 4 (2–6) | 4 (2–6) | 5 (3–9) |
| Current treatment used for flare management by BSA involvement, n | 1,172 | 1,049 | 123 |
| Topical onlya, n (%) | 860 (73.4) | 777 (74.1) | 83 (67.5) |
| cSysb,c, n (%) | 297 (25.3) | 258 (24.6) | 39 (31.7) |
| Biologicsc, n (%) | 15 (1.3) | 14 (1.3) | 1 (0.8) |
| Current treatment by BSA involvement, n | 1,988 | 1,721 | 267 |
| Topical corticosteroids, n (%) | 1,564 (78.7) | 1,346 (78.2) | 218 (81.6) |
| Topical calcineurin-inhibitors, n (%) | 506 (25.5) | 430 (25.0) | 76 (28.5) |
| Systemic corticosteroidsc, n (%) | 379 (19.1) | 323 (18.8) | 56 (21.0) |
| Systemic immunosuppressants (oral/injected)c, n (%) | 240 (12.1) | 194 (11.3) | 46 (17.2) |
| Biologicsc, n (%) | 46 (2.3) | 39 (2.3) | 7 (2.6) |
No missing data imputation was applied. Only observed data were used for each question.
Topical corticosteroids, topical calcineurin inhibitors.
Systemic corticosteroids, systemic immunosuppressants.
Systemic and biologics treatment could have been used in combination with topicals or as monotherapy.
BSA: body surface area; cSys: conventional systemic; IQR: interquartile range; SD: standard deviation.
The most frequently used AD therapies in both BSA groups were topical monotherapies (69.69%), followed by cSys (28.1%) (including corticosteroids (16%) and immunosuppressants (12.1%)), and biologics (2.3%). The mean number of flares over the previous year was higher in patients with BSA ≥ 10% than BSA < 10% (Table I). Most patients, independent of BSA involvement, managed flares using topicals (73.4%), followed by cSys (25.3%), and biologics (1.3%). Patients with BSA ≥ 10% used fewer topical monotherapies (67.5% vs. 74.1%) and a greater number of cSys treatments (31.7% vs. 24.6%) than patients with BSA < 10% during a flare.
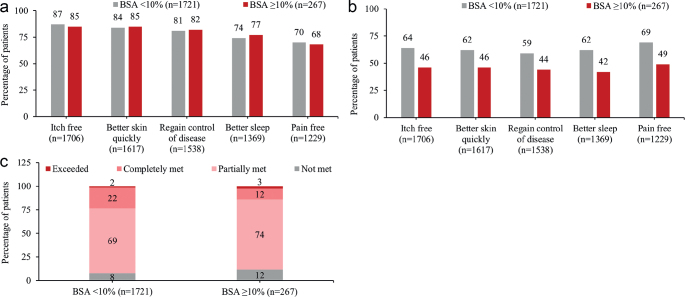
Regardless of BSA involvement, adult patients identified being itch free, achieving better skin quickly, regaining control of disease, better sleep, and being pain free as the most important treatment expectations (Fig. 1a). The most important treatment expectations were met more frequently in patients with BSA < 10% (Fig. 1b). Overall, 75% of patients in this analysis reported that their treatment expectations were only partially met or not at all met. Patients with BSA ≥ 10% were less likely to report their expectations were completely met (Fig. 1c).
Fig. 1.
Patient experience of atopic dermatitis treatment according to disease severity (body surface area; BSA). (a) Treatment goals considered quite or very important by participants. (b) The proportion of patients indicating their treatment expectations were met for treatment goals considered quite/very important. (c) Extent to which expectations were met overall.
The main reason for discontinuing prior AD treatments was insufficient skin clearance in 31.9% of patients. Other reasons for discontinuing prior treatments included physician recommendation (26.3%), the effect not being long-lasting (26.5%), and slow onset of efficacy of the drug action (24.9%). When patients were asked to report on the reasons for discontinuing treatments according to drug class, topicals were discontinued mainly due to insufficient skin clearance (35.5%), cSys treatments mainly for physician-recommended treatment changes (29.4%), and biologicals mainly due to the slow onset of efficacy (32.3%).
DISCUSSION
In this real-world cross-sectional adult patient survey, the most commonly used therapy for AD including flare management was topical monotherapy for both BSA < 10% and BSA ≥ 10% groups. The use of topical monotherapy was lower in study participants with BSA ≥ 10% vs. BSA < 10%, and their use of systemic treatments was higher, as recommended (3, 4, 7). The dominance of topical monotherapy for treatment even in patients with BSA ≥ 10% does not align with current guidelines for systemic therapy in moderate/severe AD (3, 9–11). These participants with BSA ≥ 10% also had more flares and were more likely to discontinue topical treatment due to a lack of skin clearance. This highlights the need for longterm systemic treatment solutions in these adult patients (6, 12).
The primary treatment expectations identified in this survey were similar to those in the literature and were independent of BSA involvement. Previously reported priority treatment expectations include itch reduction and healing of skin lesions (1), which may be challenging to meet if systemic therapies are under-utilized. For adequate control of moderate/severe AD, a personalized treatment strategy considering systemic treatment options, BSA involvement, flare frequency, and QoL is recommended (3, 4, 6, 7, 12).
In this study, the majority of adult patients’ treatment expectations were not met or only partially met. A higher proportion of patients with BSA ≥10% indicated their treatment expectations were unmet; this could be related to inadequate control of symptoms with topical monotherapy. Treatment dissatisfaction and even failure in AD are well documented (5, 6, 8, 13), and have been associated with inappropriate therapy choices (13), professional competence (5), poor patient compliance (8, 13), inadequate clinical improvement, adverse events, failure to relieve impairment, and treatment resistance (6). The lack of disease control in patients who experience barriers to Janus kinase 1 (JAK) inhibitors and biologics is evident1,2, and a significant proportion of patients who would be eligible for systemic therapy are not receiving them2. The unmet needs of adult patients with moderate/severe AD may require different strategies, including conventional therapies1, combination therapies (14) and systemic treatments2 (6, 12).
Limitations
Limitations of this study include the low number of adult patients using biologics, self-reported BSA involvement/AD diagnosis, and the selection and information biases. The survey was conducted in 2019, so may not reflect the current treatment environment.
Conclusion
The results of this study highlight the current unmet medical needs in AD management. This is especially so for adult patients experiencing barriers to JAK inhibitors and biologics with moderate/severe AD whose priority goals of itch relief and skin improvement were not met with topical therapy. There is a need to consider treatment options beyond topicals, recognizing that there is under-treatment with systemic options and missed opportunities to prevent disease progression.
ACKNOWLEDGEMENTS
This study was funded by Eli Lilly and Company. The authors would like to acknowledge Clare Koning and Sheridan Henness (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this publication, funded by Eli Lilly and Company.
IRB approval status: This study conformed to market research codes of conduct.
Conflicts of interest
MA is/has served as a consultant or paid speaker for AbbVie, Almirall, Amgen, Beiersdorf, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Galderma, GSK, Janssen-Cilag, Leo, Medac, Merck, MSD, Novartis, Pierre-Fabre, Pfizer, Sanofi, Trevi, UCB, and XenoPort. AC has been an advisor, speaker, and/or consultant and/or has participated in clinical studies for AbbVie, Amgen, Eli Lilly and Company, Janssen, Leo Pharma, Novartis, Pfizer, Sanofi, and UCB Pharma. AP has acted as advisor or speaker for Lilly, Abbvie, Leo, Sanofi, Novartis, Almirall, Jansen, UCB, and La-Roche Posay. JS has acted as an investigator and consultant for Abbvie, Léo-Pharma, Lilly, Novartis, Pierre Fabre, and Sanofi. CM is a contractor for HaaPACS. MdeBW has served as a principal investigator and/or advisory board member for Sanofi-Genzyme, Regeneron, AbbVie, Pfizer, Leo Pharma, Eli Lilly, UCB Galderma, Almirall, and Janssen. CS, MG, NT, and SG are employees and minor shareholders of Eli Lilly and Company.
Footnotes
Brown H, Giannakopoulou G, King A, Hamdan H, Kamaruddin A. Assessing the barriers to prescribing advanced therapies to eligible atopic dermatitis patients. Abstract retrieved from 31st EADV Congress Milan, 7–10 Sept 2022.
Heratizadeh A, Mempel M, von Kiedrowski R, Hagl S, Fritz B, Werfel T. Identification of candidates for systemic therapy in adult patients with atopic dermatitis in Germany: a multicenter study. Abstract from 31st EADV Congress Milan, 7–10 Sept 2022.
REFERENCES
- 1.Augustin M, Langenbruch A, Blome C, Gutknecht M, Werfel T, Ständer S, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol 2020; 34: 142–152. [DOI] [PubMed] [Google Scholar]
- 2.Calzavara Pinton P, Cristaudo A, Foti C, Canonica GW, Balato N, Costanzo A, et al. Diagnosis and management of moderate to severe adult atopic dermatitis: a Consensus by the Italian Society of Dermatology and Venereology (SIDeMaST), the Italian Association of Hospital Dermatologists (ADOI), the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC), and the Italian Society of Allergological, Environmental and Occupational Dermatology (SIDAPA). G Ital Dermatol Venereol 2018; 153: 133–145. [DOI] [PubMed] [Google Scholar]
- 3.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol 2018; 32: 657–682. [DOI] [PubMed] [Google Scholar]
- 4.Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, Bruin-Weller M, et al. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad of Dermatol and Venereol 2020; 34: 2717–2744. [DOI] [PubMed] [Google Scholar]
- 5.Schmitt J, Csötönyi F, Bauer A, Meurer M. Determinants of treatment goals and satisfaction of patients with atopic eczema. J Dtsch Dermatol Ges 2008; 6: 458–465. [DOI] [PubMed] [Google Scholar]
- 6.Boguniewicz M, Alexis AF, Beck LA, Block J, Eichenfield LF, Fonacier L, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract 2017; 5: 1519–1531. [DOI] [PubMed] [Google Scholar]
- 7.Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol 2017; 77: 623–633. [DOI] [PubMed] [Google Scholar]
- 8.Augustin M, Radtke MA, Zschocke I, Blome C, Behechtnejad J, Schäfer I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res 2009; 301: 561–571. [DOI] [PubMed] [Google Scholar]
- 9.Dhadwal G, Albrecht L, Gniadecki R, Poulin Y, Yeung J, Hong C-H, et al. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section IV: treatment options for the management of atopic dermatitis. J Cutan Med Surg 2018; 22: 21S–9S. [DOI] [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence (NICE) . Frequency of application of topical corticosteroids for atopic eczema. Technology appraisal guidance 2004. London: NICE. [Google Scholar]
- 11.Rubel D, Thirumoorthy T, Soebaryo RW, Weng SCK, Gabriel TM, Villafuerte LL, et al. Consensus guidelines for the management of atopic dermatitis: An Asia-Pacific perspective. J Dermatol 2013; 40: 160–171. [DOI] [PubMed] [Google Scholar]
- 12.Fougerousse A-C, Jacobzone C, Mery-Bossard L, Reguiai Z, Droitcourt C, Taieb C, et al. Use of systemic medications for treating adult atopic dermatitis in France: results of a practice survey. Clin Cosmet Investig Dermatol 2021; 14: 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol 2019; 12: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov 2022; 21: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]