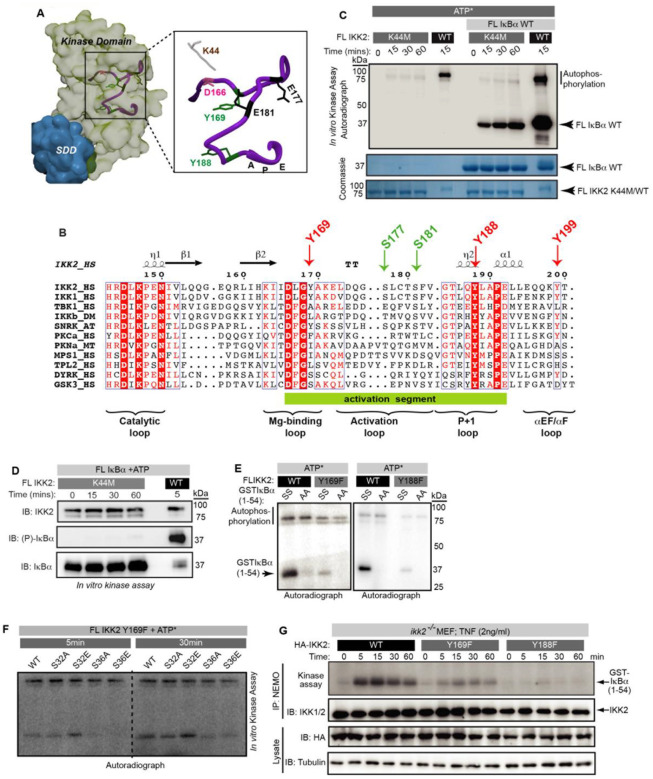
Figure 3: Dual-specific autophosphorylation is critical for IKK2’s function:
(A) Position of canonically important residues in the AL (purple ribbon) of IKK2-KD are shown in the context of its structure (PDB ID 4E3C; surface representation; KD is shown light green with respect to the SDD in teal). Autophosphorylated tyrosine residues identified in this study are shown in green. (B) Alignment of the activation segment sequences of different IKK family kinases. Conservation of Tyr at the DFG+1 (DLG in case of IKK1 and IKK2) is retained only in a stress response related plant kinase SnRK2, but not in other IKK homologues or any known dual specificity kinases, e.g., DYRK and GSK3β (both contain Ser at that position). Tyr188 (204 in PKA) is conserved. (C) Comparison of substrate and autophosphorylation activities of FL IKK2 WT and K44M mutant in an in vitro radioactive kinase assay performed with or without FL IκBα WT as the substrate. (D) Residue level specificity of phosphorylation by the FL IKK2 K44M was analysed using an antibody that specifically recognizes phospho-S32/36 of IκBα. (E) Radioactive in vitro kinase assay performed with IKK2 Y169F and Y188F mutants using GST (1–54) IκBα WT and AA as substrates. (F) Radioactive in vitro kinase assay performed with IKK2 Y169F mutant with a range of GST (1–54) IκBα mutants where the substrate phosphorylation signal is abolished in case of S36 A/E IκBα. (G) Severe reduction in TNFα-induced IKK activity in ikk2−/− MEF-3T3 cells reconstituted with mutant (Y169F and Y188F) IKK2s compared to wild-type. Kinase assay was performed with IKK immunoprecipitated (IP-ed) with anti-NEMO antibody from whole cell extract (n=2).