Highlights
-
•
Drug use, HIV symptoms, and memory were assessed among 48 HIV+ daily cannabis users.
-
•
More years HIV+ predicted worse memory proficiency controlling for chronological age.
-
•
Cannabis use and HIV symptomatology were unrelated to memory proficiency.
Keywords: HIV, Cannabis, Associative learning, Aging, Cognitive decline
Abstract
Background
Antiretroviral medications have increased the lifespan of persons living with HIV (PLWH) thereby unmasking memory decline that may be attributed to chronological age, HIV symptomatology, HIV disease chronicity, and/or substance use (especially cannabis use which is common among PLWH). To date, few studies have attempted to disentangle these effects. In a sample of daily cannabis-using PLWH, we investigated whether hippocampal memory function, assessed via an object-location associative learning task, was associated with age, HIV chronicity and symptom severity, or substance use.
Methods
48 PLWH (12.9 ± 9.6 years since HIV diagnosis), who were 44 years old on average (range: 24–64 years; 58 % male) and reported daily cannabis use (recent use confirmed by urinalysis) completed the study. We assessed each participant's demographics, substance use, medical history, current HIV symptoms, and hippocampal memory function via a well-validated object-location associative learning task.
Results
Multiple regression analyses found that living more years since HIV+ diagnosis predicted significantly worse associative learning total score (r=-0.40) and learning rate (r=-0.34) whereas chronological age, cannabis-use characteristics, and recent HIV symptom severity were not significantly related to hippocampal memory function.
Conclusions
In daily cannabis-using PLWH, HIV chronicity was related to worse hippocampal memory function independent from cannabis use, age, and HIV symptomatology. Object-location associative learning performance could serve as an ‘early-warning’ metric of cognitive decline among PLWH. Future research should examine longitudinal changes in associative learning proficiency and evaluate interventions to prevent hippocampal memory decline among PLWH. ClinicalTrials.gov: NCT01536899.
1. Introduction
Persons living with human immunodeficiency virus [HIV] (PLWH) exhibit numerous symptoms associated with both viral infection and antiretroviral treatment. Contemporary antiretroviral medications improve longevity for PLWH, such that HIV is a chronic condition to be managed, thereby unmasking aging-related comorbidities (Deeks et al., 2013; Sacktor and Robertson, 2014). Among these is cognitive decline (Baldewicz et al., 2004; Coban et al., 2017; Schouten et al., 2011), as HIV is brain-penetrant and antiretroviral medications do not prevent all neurocognitive sequelae (Rubin et al., 2017; Valcour et al., 2011). PLWH exhibit deficits in several cognitive domains, especially memory function, relative to healthy controls (Heaton et al., 2010, 2011; Ranganathan and D'Souza, 2006; Solowij and Battisti, 2008). Furthermore, cognitive deficits among PLWH are associated with older chronological age (Tan et al., 2013) and greater symptom burden (Reger et al., 2002).
PLWH use cannabis at higher rates than the general United States population (Mimiaga et al., 2013; Pacek et al., 2018). In addition to recreational cannabis use, many PLWH report using cannabis for therapeutic motives to alleviate common symptoms of HIV or side effects of antiretroviral medications, e.g., pain, fatigue, sleep disturbance, depression, anxiety, poor appetite, and nausea (Dansak, 1997; Fairfield et al., 1998; Fogarty et al., 2007; Furler et al., 2004; Greenwald et al., 2021; Woolridge et al., 2005). Whereas ‘cannabis self-medication’ to alleviate HIV symptoms does occur, there may be detrimental effects on cognitive function either via direct cannabis effects or interactions with antiretroviral medications and HIV pathophysiology. For example, chronic cannabis users exhibit decrements in episodic spatial memory performance (Schoeler and Bhattacharyya, 2013). Among PLWH, earlier onset of regular cannabis use was associated with worse memory function compared to non-cannabis users (Skalski et al., 2018). Thus, duration of regular cannabis use could exacerbate normative age-related memory decline among PLWH. Further, cannabis use could adversely interact with viral load or severity of HIV symptoms that, in turn, affect memory function. Disentangling these alternative explanations is challenging (Skalski et al., 2018). The only study to examine these relationships found that near-daily cannabis use was associated with impaired delayed memory recall among symptomatic HIV+ patients compared to asymptomatic HIV+ and HIV- patients (Cristiani et al., 2004). Age-related memory effects are unlikely as those three groups were well-matched for age. However, that study did not determine whether cannabis use (duration, frequency, or severity) or HIV disease chronicity (years living HIV+) may have differentially contributed to decrements in memory function, and there is a dearth of evidence on these issues. Investigation of the potentially additive effects of cannabis use and HIV disease burden on normative age-related memory decline among PLWH is clinically significant because antiretroviral medications are so effective now that HIV has become a chronic, managed medical condition.
The present study investigated the effects of HIV, chronological aging, or cannabis use on object-location associative learning task performance among PLWH. Object-location associative learning is dependent on several sub-processes including sustained attention, memory encoding, and memory retrieval. Prior research indicates that associative learning task performance reliably activates the hippocampus (Belchior et al., 2014; Castelo et al., 2006; Ravishankar et al., 2019) and that associative learning proficiency is positively correlated with the magnitude of hippocampus activation (Stanley et al., 2017; Suthana et al., 2015). Object-location memory tasks, like the one used herein, integrate “what” and “where” features of hippocampal memory function (Bachevalier and Nemanic, 2008; Kesner and Rolls, 2015; Kessels et al., 2001). In addition to the hippocampus, a distributed network of corticolimbic brain structures has been implicated in associative learning, including the parahippocampus, cingulate cortex, and prefrontal cortical (PFC) regions (Bähner et al., 2015; Benchenane et al., 2011; Harms et al., 2013; Woodcock et al., 2015). Each of these brain regions is densely populated with CB1 receptors, the binding target of ∆9-tetrahydrocannabinol (THC), which has been shown to alter memory function (Busquets‐Garcia et al., 2015; Davies et al., 2002; Herkenham et al., 1990; Wise et al., 2009). Indeed, prior research indicates that associative learning proficiency is impaired among PLWH (Lovejoy and Suhr, 2009; Sacktor and Robertson, 2014; Watkins and Treisman, 2015) and separately, cannabis users (Lundqvist, 2005; Solowij and Battisti, 2008; Zink et al., 2001), but research is needed to investigate the potentially additive effects of aging, HIV symptoms and chronicity, and cannabis use on hippocampal memory function (Meade et al., 2019).
In this study of HIV+ daily cannabis users, we examined whether chronicity variables (current age, years living with HIV diagnosis, age at onset of cannabis use, years using cannabis), current HIV symptom severity, or past-month cannabis (and other substance) use frequency modulated object-location associative learning task performance. We hypothesized that being older (current age) and living more years with HIV diagnosis would be related to worse associative learning proficiency.
2. Methods
2.1. Participants
The Wayne State University IRB approved all procedures. This study is registered at ClinicalTrials.gov (NCT01536899). From May 2012 through April 2015, PLWH from ages 18 to 70 years old were recruited in the university-affiliated Infectious Disease Clinic, regardless of race, ethnicity, or biological sex. Using word-of-mouth referral and advertisements posted within the clinic, treatment team members recruited candidates for this study. After written informed consent, candidates were verified sober (expired breath alcohol ≤ 0.02 %; AlcoSensor Intoximeter) and provided a urine sample (assayed for recent substance use), and completed clinical assessments and an object-location associative learning task described below.
Candidates were included in this study if he/she reported daily cannabis use over the past 90 days, provided a THC-positive urine sample, and were HIV+. Candidates were excluded from this study if he/she exhibited impaired verbal intelligence (score < 80; (Zachary, 1991)) or reported a history of neurological injury/disease. Other substance use was not exclusionary, so long as he/she was not intoxicated and could provide informed consent. Each study session lasted 3–4 h and participants were compensated $100 for completing the visit. Throughout this study, cannabis could be used legally in the State of Michigan under a medical certification law.
2.2. Clinical assessments
HIVchronicity and treatment. Patients self-reported the year they were diagnosed with HIV which, subtracted from current age, was used to estimate number of years living with an HIV+ diagnosis.
HIV-related symptoms. Participants rated past 90-day severity of 24 symptom items, each using a 0–3 scale (0=not at all, 1=slightly, 2=somewhat, 3=severe): pain, depression, anxiety, poor appetite, weight change, poor sleep, fatigue/lack of energy, memory loss, cough, shortness of breath, fever, night sweats, nausea, vomiting, constipation, diarrhea, tingling in extremities, numbness in extremities, muscle weakness, tremor, headache, not interested in sex, slurred speech, and vision problem. We computed a symptom count score (number of symptoms endorsed at non-zero levels), mean severity score across these 24 items, and number of symptoms self-treated with cannabis use.
The Patient Health Questionnaire (Spitzer et al., 1999) was administered to obtain scores for its six subscales: Somatic (13 items [e.g., back pain, headaches, shortness of breath] scored “not bothered at all”, “bothered a little”, or “bothered a lot” [0–2]), Depression (9 items [e.g., feeling depressed, little interest in doing things, feeling tired/little energy] scored “not at all”, “several days”, “more than half the days”, or “nearly every day” [0–3]), Anxiety (4 items [e.g., have you had an anxiety attack? Do some occur out of the blue?] scored absent/present [0–1] and 7 items scored the same as Depression subscale), Eating disorder problems (8 items [e.g., feel that you can't control what or how much you eat?] scored absent/present [0–1]), Alcohol problems (6 items [e.g., drank alcohol, were high or hung over when you had responsibilities] scored absent/present [0–1]) and 1 item scored “not difficult at all”, “somewhat difficult”, “very difficult”, or “extremely difficult” [0–3]), and Stressors (10 items scored the same as Somatic subscale).
The Beck Depression Inventory-II (BDI-II; (Beck et al., 1996)) assesses 21 neurovegetative symptoms of depression using a Likert scale scored from 0 ‘symptom absent’ to 3 ‘symptom is severe’. Items include feelings of ‘sadness’ rated from “I do not feel sad” (0) to “I am so sad or unhappy that I can't stand it” (3). Scores range from 0 to 63 with higher scores reflecting more severe past two-week depression symptom severity. A score of 14 is considered threshold for mild depression.
Substance use history. A standardized self-report battery developed and employed in our lab, the Drug History and Use Questionnaire ((Moses and Greenwald, 2019); available on request) was used to assess current and historical use of nicotine, alcohol, cannabis, cocaine, sedatives (prescribed and nonmedical), and opioids (prescribed and nonmedical). For each substance, participants were asked to indicate age at initial use, age at regular use (at least weekly), age at daily use, frequency of past 90-day use, and route/s of administration. Years of cannabis use, i.e., duration, was calculated as the difference between current age and age at initial use. Participants were asked to report the percentage of time over the past 90 days they “used marijuana for symptom relief” (0–100 scale; 100 %=entirely therapeutic use, 0 %=entirely recreational use).
Cannabis use disorder severity. A trained masters-level clinical psychologist, supervised by LHL, administered the Cannabis section from the Structured Clinical Interview for DSM-IV (First et al., 1995), revised to assess for DSM-5 criteria. Symptom data were coded and summed to establish presence and severity (0=absent, 1=mild, 2=moderate, 3=severe) of DSM-5 Cannabis Use Disorder (CUD).
2.3. Associative learning task
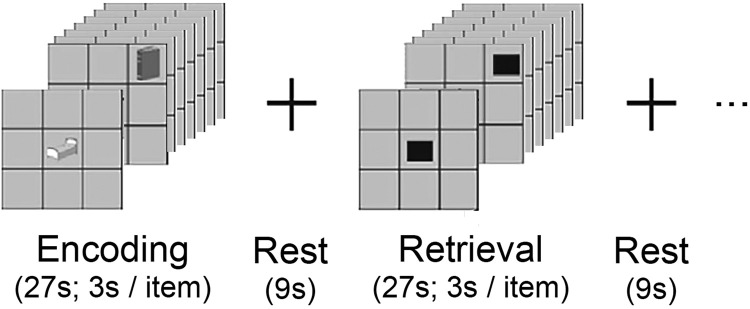
Participants performed a computerized task (adapted from (Büchel et al., 1999)) and implemented in studies at our site (Diwadkar et al., 2015a, 2008). A cartoon depiction is shown in Fig. 1. Across 8 task blocks, participants were instructed to learn associations between 9 common objects [from a standardized battery (Snodgrass and Vanderwart, 1980)] and its location in a 3 × 3 grid. Each task block was subdivided into periods of object-location memory encoding and location-cued memory retrieval (separated by a 9‑sec ‘rest’ period). During encoding, objects were presented in sequential randomized order (3 s/object) in their associated grid location. During retrieval, subjects were prompted by a black square in the grid location to verbalize the object associated with each spatial location. Participants were asked to respond “no” if they could not recall the object in a particular location. Retrieval accuracy (number of object-location associations recalled correctly) in each task block was recorded.
Fig. 1.
Cartoon depiction of the associative learning task. The associative learning task consisted of 8 repetitions (task blocks) of memory encoding, rest, cued retrieval, and rest. Each participant was instructed to learn 9 object-location associations across 8 task blocks. During memory encoding, 9 common objects, depicted in grayscale line drawings, were presented serially for 3 s, one in each unique 3 × 3 grid location (pseudo-random order). During each rest period, a static fixation cross was presented in the center of the screen for 9 s. During cued retrieval, 9 black squares were presented serially, one in each unique 3 × 3 grid location (pseudo-random order). The location cues (black squares) prompted the subject to recall and verbalize the name of the object associated with that location.
2.4. Data analysis
Analyses were performed using SPSS v.26 (IBM; Armonk, NY). Chi-square tests were used to evaluate group differences for categorical variables (e.g., investigating differential distribution of group membership, i.e., demographic variables such as biological sex [males vs. females], across tertile groups of ‘years living with HIV+ diagnosis’; see Table 1), and Analyses of Variance (ANOVAs) or Covariance (ANCOVAs) were used for continuous measures (e.g., psychiatric symptom severity). Pearson correlations were computed to measure bivariate associations. Means ± one standard deviation (SD) are presented for descriptive purposes. All analyses used significance levels of p<.05. Huynh-Feldt correction was used for violations of sphericity in repeated measures analyses.
Table 1.
Participant characteristics.
| Years Living With HIV+ Diagnosis |
Omnibus | Hi vs. Low | ||||
|---|---|---|---|---|---|---|
| Overall (N = 48) | <9 yrs (n = 16) | 9–15 yrs (n = 16) | 15+ yrs (n = 16) | F or Χ2 | F or Χ2 | |
| Demographics | ||||||
| Sex (% Male) | 58.4 % | 43.8 % | 75.0 % | 56.3 % | 3.26 | 0.50 |
| Race (% Black) | 91.7 % | 87.5 % | 100.0 % | 87.5 % | 2.18 | 0.00 |
| Age (yrs) | 43.6 (10.3) | 39.6 (10.7) | 43.4 (11.3) | 47.9 (7.0) | 2.84¶ | 6.73* |
| Economics | ||||||
| Past-month: Income ($) | 1241.1 (1002.3) | 1245 (1184) | 1381 (1105) | 1098 (694) | 0.57 | 0.15 |
| Past-month: Cannabis expenditures ($) | 156.4 (161.1) | 181 (161) | 127 (113) | 161 (202) | 0.40 | 0.26 |
| Past-month:% Income on cannabis (%) | 16.3 (17.1) | 17.9 (15.3) | 11.9 (12.3) | 19.1 (22.4) | 0.64 | 0.17 |
| HIV diagnosis | ||||||
| Age at HIV diagnosis (yrs) | 30.7 (9.6) | 35.0 (10.1) | 30.6 (10.7) | 26.4 (6.0) | 3.50* | 8.51⁎⁎ |
| Delay (HIV Dx - cannabis initiation; yrs) | 15.7 (12.1) | 21.8 (11.6) | 16.4 (12.0) | 9.0 (9.6) | 5.30⁎⁎ | 11.46⁎⁎ |
| Years since HIV+ diagnosis | 13.0 (7.8) | 4.8 (2.7) | 12.5 (2.3) | 21.9 (4.7) | 104.40⁎⁎⁎ | 163.94⁎⁎⁎ |
| Depression | ||||||
| Recent symptoms (BDI-II; Total) | 15.3 (10.9) | 15.2 (12.6) | 13.8 (8.9) | 16.8 (11.6) | 0.28 | 0.14 |
| % Clinical Depression (BDI-II ≥ 14) | 62.5 % | 56.3 % | 62.5 % | 68.8 % | 0.53 | 0.53 |
| Symptom severity scores (0–3) | ||||||
| Pain | 1.9 (1.1) | 1.9 (1.1) | 2.1 (1.2) | 1.6 (1.0) | 1.07 | 0.74 |
| Depression | 1.3 (1.2) | 1.4 (1.4) | 1.0 (1.2) | 1.5 (1.0) | 0.79 | 0.09 |
| Anxiety | 1.1 (1.2) | 1.6 (1.3) | 1.0 (1.3) | 0.9 (1.0) | 1.57 | 3.01¶ |
| Poor appetite | 1.5 (1.1) | 1.8 (1.1) | 1.3 (1.2) | 1.5 (1.2) | 0.78 | 0.41 |
| Poor sleep | 1.5 (1.3) | 1.7 (1.4) | 1.6 (1.3) | 1.1 (1.2) | 1.06 | 1.72 |
| Fatigue | 1.4 (1.2) | 1.4 (1.3) | 1.2 (1.1) | 1.5 (1.2) | 0.31 | 0.02 |
| Memory loss | 0.7 (0.9) | 0.8 (0.9) | 0.3 (0.8) | 1.1 (1.1) | 3.09¶ | 0.48 |
| Mean | 1.9 (0.4) | 1.9 (0.4) | 1.9 (0.3) | 1.8 (0.4) | 0.41 | 0.57 |
| Patient Health Questionnaire (PHQ) subscale severity | ||||||
| Somatic | 7.3 (4.8) | 7.6 (5.2) | 7.0 (4.7) | 7.2 (4.7) | 0.05 | 0.05 |
| Depression | 14.2 (9.4) | 15.1 (11.5) | 13.8 (8.5) | 13.8 (8.5) | 0.11 | 0.15 |
| Anxiety | 4.67 (4.5) | 5.3 (5.0) | 4.9 (4.6) | 3.8 (4.0) | 0.44 | 0.82 |
| Eating behavior problems | 0.6 (1.0) | 0.6 (1.1) | 0.9 (1.3) | 0.3 (0.4) | 1.87 | 1.63 |
| Alcohol | 1.1 (1.2) | 1.4 (1.6) | 0.9 (0.8) | 1.0 (0.9) | 0.88 | 0.88 |
| Stressors | 8.6 (5.0) | 8.8 (4.9) | 8.5 (5.4) | 8.6 (5.0) | 0.01 | 0.01 |
| Cannabis (MJ) use | ||||||
| Age at initial cannabis use (yrs) | 15.0 (5.3) | 13.3 (4.8) | 14.2 (3.4) | 17.4 (6.4) | 3.08¶ | 4.39* |
| Age at daily cannabis use (yrs) | 24.0 (9.8) | 22.1 (6.7) | 21.3 (5.9) | 28.6 (13.5) | 2.95¶ | 3.02¶ |
| CUD symptoms (#) | 5.1 (2.0) | 4.4 (2.3) | 5.3 (1.5) | 5.5 (2.0) | 1.51 | 2.17 |
| CUD severity (0–3) | 2.0 (0.9) | 1.8 (0.8) | 1.9 (0.9) | 2.2 (1.0) | 0.90 | 1.81 |
| Therapeutic use of cannabis (%) | 59.0 % | 63.1 % | 52.7 % | 61.3 % | 1.02 | 1.27 |
| Other substance use | ||||||
| Alcohol (% daily use) Any past 90-day cocaine use (%) Cocaine (% UDS+) |
20.8 % 12.5 % 16.7 % |
18.8 % 6.3 % 18.8 % |
12.5 % 14.3 % 0.0 % |
31.3 % 37.5 % 31.3 % |
1.77 5.74¶ 5.70¶ |
0.67 2.67 0.67 |
| Any past 90-day opioid use (%) Opioids (% UDS+) |
43.8 % 18.8 % |
37.5 % 6.3 % |
62.5 % 25.0 % |
31.3 % 25.0 % |
3.56 2.46 |
0.14 2.13 |
| Benzodiazepines (% UDS+) | 10.4 % | 12.5 % | 6.3 % | 12.5 % | 0.45 | 0.00 |
| Daily cigarette smokers (%) Daily cigarette use (#) among users |
64.6 % 12.1 (7.2) |
75.0 % 10.6 (7.2) |
56.2 % 14.3 (8.5) |
62.5 % 11.9 (6.3) |
1.28 0.68 |
0.58 0.21 |
Notes: Cell entries reflect group mean (SD) or percentage. BDI-II, Beck Depression Inventory-II; CUD, cannabis use disorder; UDS, urine drug screen; Dx, diagnosis. The 'Omnibus' column refers to one-way ANOVA or chi-square tests of the main effects of ‘years living with HIV+ diagnosis’ stratified into tertile groups (each n = 16) on each variable of interest (e.g., cannabis use variables). The 'High vs. Low' column refers to statistical tests contrasting only the ‘high’ vs. ‘low’ tertile groups (ignoring the ‘moderate’ tertile group) for 'years since HIV+ diagnosis' on each variable of interest. Significant differences noted:.
p < .10;.
p < .05;.
p < .01;.
p < .001.
Two metrics for associative learning proficiency were calculated: total learning score, i.e., sum of correctly recalled object-location associations across the 8 task blocks; and associative learning rate, i.e., learning rate across trials. Associative learning typically exhibits negatively-accelerated growth (Banyai et al., 2011). Thus, we characterized associative learning rate by fitting a negatively-accelerated function ([proportion correct = ] for each participant. The single unconstrained parameter in that function, k, reflects associative learning rate (higher k values indicate more rapid learning).
Bivariate correlations were first computed between learning scores (total learning score range, 0 to 72; and learning rate, k) and demographic factors (age, sex), HIV history (age at diagnosis, time since diagnosis), HIV symptom severity scores, cannabis-use characteristics (e.g., age at onset, past 90-day frequency of use, duration of use, DSM-5 severity), and recent depression symptoms (BDI-II total score). Variables hypothesized to exhibit a relationship with associative learning were evaluated as predictors in two multivariate stepwise linear regression models predicting total learning score and learning rate, separately.
3. Results
3.1. Participant characteristics
Forty-nine participants completed the associative learning task, though one was excluded for being study non-compliant. On average, the 48 participants included in this study were 43.6 ± 10.3 years old (range: 24–64 years old), mostly male (58 %), and predominantly Non-Hispanic Black (91.7 %; Table 1). Participants reported initiating cannabis use at 15.0 ± 5.3 years old and transitioning to daily use ∼9 years later (24.0 ± 9.8 years old). Participants were diagnosed with HIV at 30.7 ± 9.6 years old. Average duration of cannabis use was ∼28 years whereas years living with HIV+ diagnosis was ∼13 years.
During the 90 days prior to screening, 10 participants (20.8 %) reported daily alcohol use. Nearly half (43.8 %) reported some past 90-day use of opioids (n = 21) whereas 6 participants (12.5 %) tested positive for cocaine. Most participants denied past 90-day use of sedatives, but 5 participants (10.4 %) tested positive for benzodiazepines. Finally, nearly two-thirds (64.6 %; n = 31) smoked cigarettes daily.
3.2. Associative learning
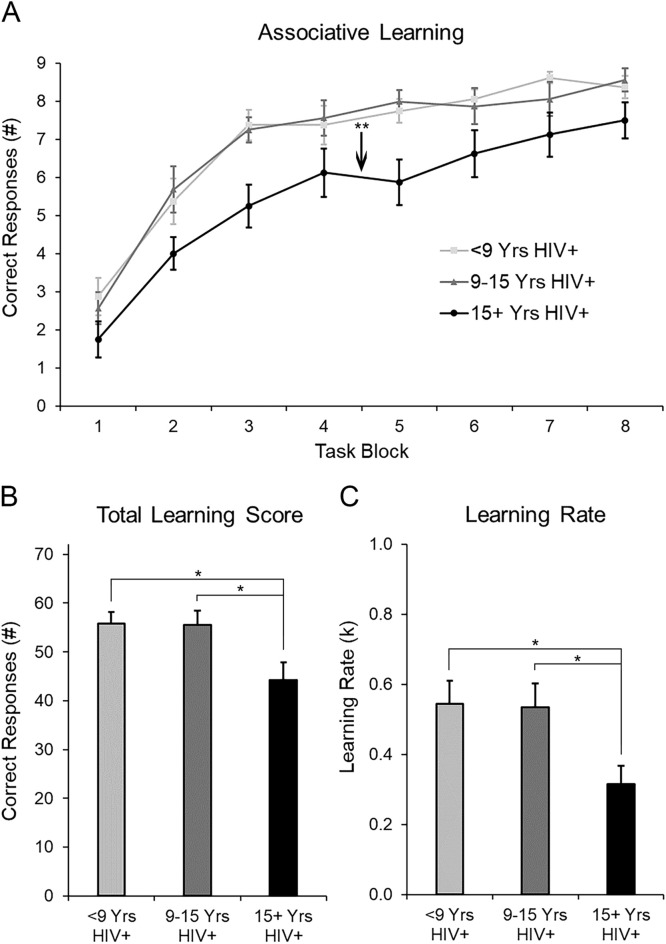
As hypothesized, learning performance was negatively accelerated across task blocks. Controlling for age, repeated measures analysis of covariance (rmANCOVA) revealed object-location associative learning improved across trials, F(7322)=12.24, p=.000. Average performance improved from ∼2 correct associations in the first task block to ∼8 correct associations (of 9 possible) in the final task block (Fig. 2A). Asymptotic learning occurred by block 5, on average, and participants learned 8.5 object-location associations (94.4 % accurate). Mean learning rate, k, was 0.47 (Fig. 2C), similar to prior studies (Diwadkar et al., 2015b, 2008). Total learning score was ∼52 correct associations (of 72 possible) or 72 % accuracy (Fig. 2B).
Fig. 2.
(A) Mean (± 1 SEM) associative learning performance across task blocks is depicted by tertiles of ‘years living with HIV+ diagnosis’. (B) Total associations learned, i.e., total learning score, is depicted by tertiles of ‘years living with HIV+ diagnosis’. (C) Learning rate (k), estimated by fitting a negatively accelerating learning function (1-e − k*block), is depicted by tertiles of ‘years living with HIV+ diagnosis’. Note: *p < .05; **p ≤ 0.01.
3.3. Predictors of associative learning
The Supplementary Table presents bivariate Pearson correlations of variables tested in the stepwise multivariate regression analyses described below.
Total learningscore. In the first regression, only one variable significantly predicted total learning score, years living with HIV+ diagnosis, accounting for 16 % of the variance (Table 2). Living more years with HIV+ diagnosis was associated with fewer associations learned. Unstandardized coefficients indicate that for each year living with HIV+ diagnosis, total object-location associations decreased by 0.67 or ∼1.3 %. Chronological age, age at HIV+ diagnosis, age at initial cannabis use, years using cannabis, DSM-5 CUD symptoms and severity, and cannabis-use frequency were not related to total learning score (ps≥.20), whereas past 90-day HIV symptom burden was ‘trend’-level (p=.08). Controlling for chronological age, a second stepwise linear regression indicated that years since HIV+ diagnosis remained a significant predictor (p<.03), confirming this relationship is not confounded by age.
Table 2.
Stepwise linear regression analysis predicting total associative learning score.
| Predictors | R2 | Adj. R2 | Unstd. B (SE) | Std. β | t | p |
|---|---|---|---|---|---|---|
| Included Variables | ||||||
| Years since HIV diagnosis | .16 | .15 | −0.67 (0.23) | −0.40 | −2.96 | .005 |
| Excluded Variables | ||||||
| Chronological age | −0.12 | −0.80 | .43 | |||
| Age at HIV diagnosis | −0.08 | −0.56 | .58 | |||
| Past 90-day HIV symptoms | .24 | 1.78 | .08 | |||
| Age at initial cannabis use | −0.15 | −1.08 | .31 | |||
| Years using cannabis | −0.02 | −0.13 | .90 | |||
| Past 90-day cannabis use | .16 | 1.16 | .25 | |||
| DSM-5 CUD total symptoms | .18 | 1.30 | .20 | |||
| DSM-5 CUD severity | −0.14 | −0.97 | .34 | |||
| BDI-II total score | −0.06 | −0.41 | .69 |
Abbreviations: CUD: cannabis use disorder; BDI-II: Beck Depressive Inventory-II.
Associative learning rate. In the second regression, only one variable significantly predicted associative learning rate, years since HIV+ diagnosis, accounting for 11 % of the variance (Table 3). Unstandardized coefficients indicate that for each year living with HIV, learning rate decreased by 2.3 %. Chronological age, age at HIV+ diagnosis, years using cannabis, DSM-5 CUD symptoms and severity, and cannabis-use frequency were not related to total learning score (ps≥.20), whereas past 90-day HIV symptom burden was ‘trend’-level (p = .08). To confirm this relationship was not confounded by chronological age, a second linear regression, controlling for age, showed that years since HIV+ diagnosis remained a significant predictor (p<.03).
Table 3.
Stepwise linear regression analysis predicting associative learning rate.
| Predictors | R2 | Adj. R2 | Unstd. B (SE) | Std. β | t | p |
|---|---|---|---|---|---|---|
| Model | ||||||
| Years since HIV diagnosis | .11 | .09 | −0.011 (0.005) | −0.34 | −2.39 | .021 |
| Excluded Variables | ||||||
| YChronological age | .06 | 0.35 | .73 | |||
| YAge at HIV diagnosis | .07 | 0.48 | .64 | |||
| YPast 90-day HIV symptoms | .25 | 1.80 | .08 | |||
| YAge at initial cannabis use | −0.21 | −1.43 | .16 | |||
| YYears using cannabis | .15 | 1.03 | .31 | |||
| YPast 90-day cannabis use | .07 | 0.47 | .64 | |||
| YDSM-5 CUD total symptoms | .18 | 1.26 | .22 | |||
| YDSM-5 CUD severity | −0.17 | −1.17 | .25 | |||
| BDI-II total score | −0.04 | −0.30 | .76 |
Abbreviations: CUD: cannabis use disorder; BDI-II: Beck Depressive Inventory-II.
3.4. Years living with HIV+ diagnosis
As years living with HIV+ diagnosis was the only significant predictor of associative learning performance, we evaluated its influence on other clinical variables by stratifying years living with HIV+ diagnosis into 3 equal groups (n = 16/tertile) and treating it as a predictor variable. Chronological age differed between tertile groups (Table 1) and thus, was included as a covariate. Controlling for current age, persons living the longest with an HIV+ diagnosis (15+ years) exhibited worse total learning and slower learning rate than those with fewer years since HIV+ diagnosis, F(2,47)=3.48, p=.04, and F(2,47)=3.99, p=.03, respectively. Relative to fewer years HIV+, PLWH 15+ years were older (∼48 vs. ∼40 years old), were diagnosed with HIV at a younger age (∼26 vs. ∼35 years old), exhibited a shorter delay between onset of cannabis use and HIV diagnosis (∼9 vs. ∼22 years), and initiated cannabis use at an older age (∼17 vs. ∼13 years old). In contrast, more years living with HIV+ diagnosis was not associated with HIV symptom severity, frequency of cannabis or other substance use, CUD symptoms, current depression symptoms, or biological sex (ps>0.05).
To evaluate whether years living with HIV+ diagnosis masked other factors that may also be related to associative learning, partial correlations re-examined bivariate relationships, controlling for years living with HIV+ diagnosis. Partial correlations indicated no significant correlations with either associative learning metric (ps>0.05; not shown).
4. Discussion
This study found that living more years HIV+ was associated with worse hippocampal memory function among a sample of 48 daily cannabis-using PLWH. Chronological age, HIV-related symptoms, and cannabis use were not significantly related to hippocampal memory function. Further, HIV disease chronicity remained significant even after controlling for chronological age. Throughout the remainder of this manuscript we theorize potential neurobiological explanations for these findings and discuss their clinical significance.
Several neurobiological factors might explain why HIV disease chronicity is related to worse hippocampal memory function. First, in animal models, HIV-1 viral protein R may damage hippocampal neurons (Huang et al., 2000; Piller et al., 1998; Torres and Noel, 2014; Wang et al., 2017), thereby directly impairing neurons thought to encode and retrieve object-location associations. Second, HIV disease chronicity could interfere with corticolimbic network engagement during memory encoding and/or retrieval processes thereby impairing proficiency. PLWH have been shown to exhibit deficits in PFC-hippocampus activation during episodic memory encoding tasks (Castelo et al., 2006; Maki et al., 2009). Specifically, HIV+ patients exhibited significantly less activation in the hippocampus and parahippocampus during memory encoding tasks compared to HIV- controls, suggesting less network engagement during memory function. Third, elevated neuroinflammatory signaling due to HIV disease could impair hippocampal memory function via disruption of long-term potentiation (Barrientos et al., 2012; del Rey et al., 2013; Murray and Lynch, 1998; Prieto and Cotman, 2017; Pugh et al., 2001; Vera et al., 2016). Fourth, more years living with HIV could worsen memory through common medical comorbidities such as obesity, diabetes, and hypertension. Unfortunately, we could not access sufficient data from participants’ medical charts to examine these hypotheses. Future research is needed to determine which neurobiological processes explain our observation that HIV disease chronicity uniquely predicts poor hippocampal memory proficiency.
Interestingly, memory proficiency was not related to Cannabis Use Disorder severity, substance use, HIV symptom severity, chronological age, or biological sex. There are possible reasons for these null results. First, by design, all participants were current daily cannabis users, which limited between-subject variability and thus, may have impeded our ability to detect cannabis-use related effects. Second, chronic cannabinoid use, which was associated with less severe HIV-related symptoms in an overlapping sample from this clinic (Greenwald et al., 2021), could have masked relationships between HIV symptom severity and memory function, which were ‘trend’-level in this study (ps=0.08). Third, previous research has shown that substance use other than cannabis can affect memory function. For example, nicotine has been shown to modulate learning and memory via acetylcholine receptors (Filbey et al., 2015; Nava et al., 2001). However, we did not find significant relationships between cigarette use frequency or chronicity and memory performance on this task. Fourth, some studies report sex differences in object/location learning performance (Cost et al., 2012) or interactions between biological sex and cannabinoid effects on associative learning (Cha et al., 2007). While our sample was well-balanced for biological sex (28 M vs. 20F), we found no evidence for sex differences in hippocampal memory function.
The clinical significance of our findings is increasingly evident as antiretroviral medications have lengthened lifespan for PLWH and refocused clinical care to managing comorbidities, including cognitive aging and its adverse impact on daily life. Declining performance on hippocampal memory tasks over time could be an objective 'early-warning' metric of HIV-related cognitive impairment (Manji et al., 2013; McArthur et al., 2004; R Norman and Basso, 2015; Sacktor and Robertson, 2014). Interestingly, participants’ ratings of the severity of ecological memory problems during the prior 3 months was unrelated to their experimental learning performance. This indicates that learning/memory proficiency should be assessed using objective methods, rather than relying on global self-reported impairment. Associative memory tasks could be integrated into screening for cognitive impairment, providing an opportunity for intervention using mnemonic training or cognitive-enhancing medications to activate visuospatial brain regions to improve or compensate for memory changes (Cody and Vance, 2016; Dresler et al., 2017).
The present study has limitations. First, our sample consisted of mostly Black participants which may limit generalizability to other groups. Second, there were no control groups (e.g., non-HIV or non-cannabis users) and thus, no conclusions can be drawn about causal relationships between memory deficits and years living with HIV. However, our homogenous sample of daily cannabis users maximized statistical power to investigate clinical relationships. Third, no other cognitive tasks were administered, which could have improved our interpretation of cognitive findings. Fourth, we did not assess other lifestyle factors (e.g., exercise) that could enhance resilience to cognitive decline (Monroe et al., 2017). Fifth, due to technical issues, anti-retroviral medication status and viral load counts were only available for the half of the sample (n = 24; 22 of whom were taking antiretroviral medications and 16 of whom exhibited viral load suppression [counts < 200]). Of note, no primary outcome variable differed between those with vs. those without data for antiretroviral medication status and viral load (ps > 0.45).
In conclusion, longer HIV disease chronicity was associated with worse hippocampal memory proficiency among daily cannabis-using PLWH. Moreover, this relationship remained significant controlling for chronological age. In contrast, HIV symptom severity and cannabis-use characteristics were not related to hippocampal memory function. Together, these findings suggest HIV disease chronicity may impair hippocampal memory function, independent of normative age-related cognitive decline, HIV symptom burden, or substance use. Future research should examine longitudinal changes in hippocampal memory proficiency and evaluate interventions to prevent hippocampal memory decline among PLWH.
Author contributions
EAW contributed to study methodology, data analysis and visualization, and authored sections of the manuscript. MKG acquired funding, and contributed to study methodology, data analysis and visualization, supervised data collection, and authored sections of the manuscript. IC and DF reviewed and edited the manuscript. JAC supervised subject recruitment and edited the manuscript. LHL supervised psychiatric screening and edited the manuscript. All authors have reviewed content and approved the final version for publication.
Author disclosures
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
Registration
This study was registered at ClinicalTrials.gov (NCT01536899).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
Study supported by National Institute on Drug Abuse (NIH R01 DA032678 to MKG, and NIH K99/R00 DA048125 to EAW), Gertrude Levin Endowed Chair in Addiction and Pain Biology (MKG), Michigan Department of Health and Human Services (Helene Lycaki/Joe Young, Sr. funds), and Detroit Wayne Integrated Health Network. These funding sources had no role in study design, data collection, analysis or interpretation of the data, writing of the report, or the decision to submit the paper for publication.
Acknowledgments
The authors thank the study participants for their contributions and lab members in the Substance Addiction Research Division at Wayne State University for their subject recruitment and data collection efforts.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2023.100189.
Appendix. Supplementary materials
References
- Bachevalier J., Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bähner F., Demanuele C., Schweiger J., Gerchen M.F., Zamoscik V., Ueltzhöffer K., Hahn T., Meyer P., Flor H., Durstewitz D. Hippocampal–dorsolateral prefrontal coupling as a species-conserved cognitive mechanism: a human translational imaging study. Neuropsychopharmacol.: Off. Public. Am. Coll. Neuropsychopharmacol. 2015;40(7):1674–1681. doi: 10.1038/npp.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldewicz T.T., Leserman J., Leserman J., Silva S.G., Petitto J.M., Golden R.N., Perkins D.O., Barroso J., Evans D.L. Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS Behav. 2004;8:345–355. doi: 10.1023/B:AIBE.0000044081.42034.54. [DOI] [PubMed] [Google Scholar]
- Banyai M., Diwadkar V.A., Erdi P. Model-based dynamical analysis of functional disconnection in schizophrenia. Neuroimage. 2011;58(3):870–877. doi: 10.1016/j.neuroimage.2011.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R.M., Frank M.G., Watkins L.R., Maier S.F. Aging-related changes in neuroimmune-endocrine function: implications for hippocampal-dependent cognition. Horm. Behav. 2012;62(3):219–227. doi: 10.1016/j.yhbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A., Brown G. Beck depression inventory–II. Psychol. Assess. 1996 [Google Scholar]
- Belchior H., Lopes-dos-Santos V., Tort A.B., Ribeiro S. Increase in hippocampal theta oscillations during spatial decision making. Hippocampus. 2014;24(6):693–702. doi: 10.1002/hipo.22260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchenane K., Tiesinga P.H., Battaglia F.P. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr. Opin. Neurobiol. 2011;21(3):475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Büchel C., Coull J., Friston K. The predictive value of changes in effective connectivity for human learning. Science. 1999;283(5407):1538–1541. doi: 10.1126/science.283.5407.1538. [DOI] [PubMed] [Google Scholar]
- Busquets-Garcia A., Desprez T., Metna-Laurent M., Bellocchio L., Marsicano G., Soria-Gomez E. Dissecting the cannabinergic control of behavior: the where matters. Bioessays. 2015;37(11):1215–1225. doi: 10.1002/bies.201500046. [DOI] [PubMed] [Google Scholar]
- Castelo J., Sherman S., Courtney M., Melrose R., Stern C. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66(11):1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Cha Y.M., Jones K.H., Kuhn C.M., Wilson W.A., Swartzwelder H.S. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav. Pharmacol. 2007;18(5–6):563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- Coban H., Robertson K., Smurzynski M., Krishnan S., Wu K., Bosch R.J., Collier A.C., Ellis R.J. Impact of aging on neurocognitive performance in previously antiretroviral-naïve HIV+ individuals on their first suppressive regimen. AIDS. 2017;31(11):1565. doi: 10.1097/QAD.0000000000001523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody S.L., Vance D.E. The neurobiology of HIV and its impact on cognitive reserve: a review of cognitive interventions for an aging population. Neurobiol. Dis. 2016;92:144–156. doi: 10.1016/j.nbd.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Cost K.T., Williams-Yee Z.N., Fustok J.N., Dohanich G.P. Sex differences in object-in-place memory of adult rats. Behav. Neurosci. 2012;126(3):457. doi: 10.1037/a0028363. [DOI] [PubMed] [Google Scholar]
- Cristiani S.A., Pukay-Martin N.D., Bornstein R.A. Marijuana use and cognitive function in HIV-infected people. J. Neuropsychiatry Clin. Neurosci. 2004;16(3):330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- Dansak D.A. Medical use of recreational drugs by AIDS patients. J. Addict. Dis. 1997;16(3):25–30. doi: 10.1300/J069v16n03_03. [DOI] [PubMed] [Google Scholar]
- Davies S.N., Pertwee R.G., Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42(8):993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Deeks S.G., Lewin S.R., Havlir D.V. The end of AIDS: HIV infection as a chronic disease. Lancet North Am. Ed. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A., Balschun D., Wetzel W., Randolf A., Besedovsky H.O. A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behav. Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Diwadkar V.A., Burgess A., Hong E., Rix C., Arnold P.D., Hanna G.L., Rosenberg D.R. Dysfunctional activation and brain network profiles in youth with obsessive-compulsive disorder: a focus on the dorsal anterior cingulate during working memory. Front. Hum. Neurosci. 2015;9:149. doi: 10.3389/fnhum.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar V.A., Burgess A., Hong E., Rix C., Arnold P.D., Hanna G.L., Rosenberg D.R. Dysfunctional activation and brain network profiles in youth with obsessive-compulsive disorder: a focus on the dorsal anterior cingulate during working memory. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar V.A., Flaugher B., Jones T., Zalanyi L., Ujfalussy B., Keshavan M.S., Erdi P. Impaired associative learning in schizophrenia: behavioral and computational studies. Cogn. Neurodyn. 2008;2(3):207–219. doi: 10.1007/s11571-008-9054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler M., Shirer W.R., Konrad B.N., Müller N.C., Wagner I.C., Fernández G., Czisch M., Greicius M.D. Mnemonic training reshapes brain networks to support superior memory. Neuron. 2017;93(5):1227–1235. doi: 10.1016/j.neuron.2017.02.003. e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield K.M., Eisenberg D.M., Davis R.B., Libman H., Phillips R.S. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Arch. Intern. Med. 1998;158(20):2257–2264. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- Filbey F.M., McQueeny T., Kadamangudi S., Bice C., Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav. Brain Res. 2015;293:46–53. doi: 10.1016/j.bbr.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. New York State Psychiatric Institute; New York: 1995. Structured Clinical Interview For DSM-IV Axis I Disorders-Patient ed. [Google Scholar]
- Fogarty A., Rawstorne P., Prestage G., Crawford J., Grierson J., Kippax S. Marijuana as therapy for people living with HIV/AIDS: social and health aspects. AIDS Care. 2007;19(2):295–301. doi: 10.1080/09540120600841930. [DOI] [PubMed] [Google Scholar]
- Furler M.D., Einarson T.R., Millson M., Walmsley S., Bendayan R. Medicinal and recreational marijuana use by patients infected with HIV. AIDS Patient Care STDs. 2004;18(4):215–228. doi: 10.1089/108729104323038892. [DOI] [PubMed] [Google Scholar]
- Greenwald M.K., Akcasu N., Baal P., Outlaw A.Y., Cohn J.A., Lundahl L.H. Cannabis and complementary/alternative self-treatment approaches for symptom management among African American persons living with HIV. AIDS Care. 2021:1–5. doi: 10.1080/09540121.2021.1998311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M.P., Wang L., Csernansky J.G., Barch D.M. Structure–function relationship of working memory activity with hippocampal and prefrontal cortex volumes. Brain Struct. Funct. 2013;218:173–186. doi: 10.1007/s00429-012-0391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R., Clifford D., Franklin D., Woods S., Ake C., Vaida F., Ellis R., Letendre S., Marcotte T., Atkinson J. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: charter study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., LeBlanc S., Corkran S.H., Duarte N.A., Clifford D.B., Woods S.P. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J. Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., De Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl Acad. Sci. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.-B., Weeks O., Zhao L.-J., Saltarelli M., Bond V.C. Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures. J. Neurovirol. 2000;6(3):202–220. doi: 10.3109/13550280009015823. [DOI] [PubMed] [Google Scholar]
- Kesner R.P., Rolls E.T. A computational theory of hippocampal function, and tests of the theory: new developments. Neurosci. Biobehav. Rev. 2015;48:92–147. doi: 10.1016/j.neubiorev.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kessels R.P., de Haan E.H., Kappelle L.J., Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res. Rev. 2001;35(3):295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- Lovejoy T.I., Suhr J.A. The relationship between neuropsychological functioning and HAART adherence in HIV-positive adults: a systematic review. J. Behav. Med. 2009;32:389–405. doi: 10.1007/s10865-009-9212-9. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol. Biochem. Behav. 2005;81(2):319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Maki P., Cohen M., Weber K., Little D., Fornelli D., Rubin L., Perschler P., Gould F., Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72(19):1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H., Jäger H., Winston A. HIV, dementia and antiretroviral drugs: 30 years of an epidemic. J. Neurol., Neurosurg. Psychiatry. 2013;84(10):1126–1137. doi: 10.1136/jnnp-2012-304022. [DOI] [PubMed] [Google Scholar]
- McArthur J.C., McDermott M.P., McClernon D., St Hillaire C., Conant K., Marder K., Schifitto G., Selnes O.A., Sacktor N., Stern Y. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch. Neurol. 2004;61(11):1687–1696. doi: 10.1001/archneur.61.11.1687. [DOI] [PubMed] [Google Scholar]
- Meade C.S., Bell R.P., Towe S.L., Chen N.k., Hobkirk A.L., Huettel S.A. Synergistic effects of marijuana abuse and HIV infection on neural activation during a cognitive interference task. Addict. Biol. 2019;24(6):1235–1244. doi: 10.1111/adb.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimiaga M.J., Reisner S.L., Grasso C., Crane H.M., Safren S.A., Kitahata M.M., Schumacher J.E., Mathews W.C., Mayer K.H. Substance use among HIV-infected patients engaged in primary care in the United States: findings from the Centers for AIDS Research Network of Integrated Clinical Systems cohort. Am. J. Public Health. 2013;103(8):1457–1467. doi: 10.2105/AJPH.2012.301162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe A., Zhang L., Jacobson L., Plankey M., Brown T., Miller E., Martin E., Becker J., Levine A., Ragin A. The association between physical activity and cognition in men with and without HIV infection. HIV Med. 2017;18(8):555–563. doi: 10.1111/hiv.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses T.E., Greenwald M.K. History of regular nonmedical sedative and/or alcohol use differentiates substance-use patterns and consequences among chronic heroin users. Addict. Behav. 2019;97:14–19. doi: 10.1016/j.addbeh.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C.A., Lynch M.A. Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age-and stress-induced impairments in long-term potentiation. J. Neurosci. 1998;18(8):2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava F., Carta G., Colombo G., Gessa G. Effects of chronic Δ9-tetrahydrocannabinol treatment on hippocampal extracellular acetylcholine concentration and alternation performance in the T-maze. Neuropharmacology. 2001;41(3):392–399. doi: 10.1016/s0028-3908(01)00075-2. [DOI] [PubMed] [Google Scholar]
- Pacek L.R., Towe S.L., Hobkirk A.L., Nash D., Goodwin R.D. Frequency of cannabis use and medical cannabis use among persons living with HIV in the United States: findings from a nationally representative sample. AIDS Educ. Prev. 2018;30(2):169–181. doi: 10.1521/aeap.2018.30.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piller S.C., Jans P., Gage P.W., Jans D.A. Extracellular HIV-1 virus protein R causes a large inward current and cell death in cultured hippocampal neurons: implications for AIDS pathology. Proc. Natl Acad. Sci. 1998;95(8):4595–4600. doi: 10.1073/pnas.95.8.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto G.A., Cotman C.W. Cytokines and cytokine networks target neurons to modulate long-term potentiation. Cytokine Grow. Fact. Rev. 2017;34:27–33. doi: 10.1016/j.cytogfr.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C.R., Fleshner M., Watkins L.R., Maier S.F., Rudy J.W. The immune system and memory consolidation: a role for the cytokine IL-1β. Neurosci. Biobehav. Rev. 2001;25(1):29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- R Norman L., Basso M. An update of the review of neuropsychological consequences of HIV and substance abuse: a literature review and implications for treatment and future research. Curr. Drug Abuse Rev. 2015;8(1):50–71. doi: 10.2174/1874473708666150309124820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M., D'Souza D.C. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology (Berl.) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- Ravishankar M., Morris A., Burgess A., Khatib D., Stanley J.A., Diwadkar V.A. Cortical-hippocampal functional connectivity during covert consolidation sub-serves associative learning: evidence for an active “rest” state. Brain Cogn. 2019;131:45–55. doi: 10.1016/j.bandc.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M., Welsh R., Razani J., Martin D.J., Boone K.B. A meta-analysis of the neuropsychological sequelae of HIV infection. J. Int. Neuropsychol. Soc. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Rubin L.H., Maki P.M., Springer G., Benning L., Anastos K., Gustafson D., Villacres M.C., Jiang X., Adimora A.A., Waldrop-Valverde D. Cognitive trajectories over 4 years among HIV-infected women with optimal viral suppression. Neurology. 2017;89(15):1594–1603. doi: 10.1212/WNL.0000000000004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N., Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr. Opin. HIV AIDS. 2014;9(6):517–520. doi: 10.1097/COH.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeler T., Bhattacharyya S. The effect of cannabis use on memory function: an update. Subst. Abuse Rehabil. 2013:11–27. doi: 10.2147/SAR.S25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten J., Cinque P., Gisslen M., Reiss P., Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. AIDS. 2011;25(5):561–575. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- Skalski L.M., Towe S.L., Sikkema K.J., Meade C.S. Memory impairment in HIV-infected individuals with early and late initiation of regular marijuana use. AIDS Behav. 2018;22:1596–1605. doi: 10.1007/s10461-017-1898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass J.G., Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J. Exp. Psychol. [Hum. Learn.] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Solowij N., Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr. Drug Abuse Rev. 2008;1(1):81–98. doi: 10.2174/1874473710801010081. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Group, P.H.Q.P.C.S., Group, P.H.Q.P.C.S. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Stanley J.A., Burgess A., Khatib D., Ramaseshan K., Arshad M., Wu H., Diwadkar V.A. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo 1 H functional magnetic resonance spectroscopy. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthana N.A., Donix M., Wozny D.R., Bazih A., Jones M., Heidemann R.M., Trampel R., Ekstrom A.D., Scharf M., Knowlton B., Turner R., Bookheimer S.Y. High-resolution 7T fMRI of human hippocampal subfields during associative learning. J. Cogn. Neurosci. 2015;27(6):1194–1206. doi: 10.1162/jocn_a_00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan I.L., Smith B.R., Hammond E., Vornbrock-Roosa H., Creighton J., Selnes O., McArthur J.C., Sacktor N. Older individuals with HIV infection have greater memory deficits than younger individuals. J. Neurovirol. 2013;19(6):531–536. doi: 10.1007/s13365-013-0209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L., Noel R.J., Jr. Astrocytic expression of HIV-1 viral protein R in the hippocampus causes chromatolysis, synaptic loss and memory impairment. J Neuroinflammation. 2014;11:53. doi: 10.1186/1742-2094-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V., Sithinamsuwan P., Letendre S., Ances B. Pathogenesis of HIV in the central nervous system. Curr. HIV/AIDS Rep. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J.H., Guo Q., Cole J.H., Boasso A., Greathead L., Kelleher P., Rabiner E.A., Kalk N., Bishop C., Gunn R.N., Matthews P.M., Winston A. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology. 2016;86(15):1425–1432. doi: 10.1212/WNL.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Santerre M., Tempera I., Martin K., Mukerjee R., Sawaya B.E. HIV-1 Vpr disrupts mitochondria axonal transport and accelerates neuronal aging. Neuropharmacology. 2017;117:364–375. doi: 10.1016/j.neuropharm.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins C.C., Treisman G.J. Cognitive impairment in patients with AIDS - prevalence and severity. HIV AIDS (Auckl.) 2015;7:35–47. doi: 10.2147/HIV.S39665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise L.E., Thorpe A.J., Lichtman A.H. Hippocampal CB(1) receptors mediate the memory impairing effects of Delta(9)-tetrahydrocannabinol. Neuropsychopharmacol.: Off. Public. Am. Coll. Neuropsychopharmacol. 2009;34(9):2072–2080. doi: 10.1038/npp.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E.A., White R., Diwadkar V.A. The dorsal prefrontal and dorsal anterior cingulate cortices exert complementary network signatures during encoding and retrieval in associative memory. Behav. Brain Res. 2015;290:152–160. doi: 10.1016/j.bbr.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Woolridge E., Barton S., Samuel J., Osorio J., Dougherty A., Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manage. 2005;29(4):358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Zachary R.A. WPS, Western Psychological Services; 1991. Shipley Institute of Living Scale. [Google Scholar]
- Zink W.E., Boyle J., Persidsky Y., Xiong H., Gendelman H.E. Model systems for assessing cognitive function: implications for HIV-1 infection and drugs of abuse. Adv. Exp. Med. Biol. 2001;493:7–27. doi: 10.1007/0-306-47611-8_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.