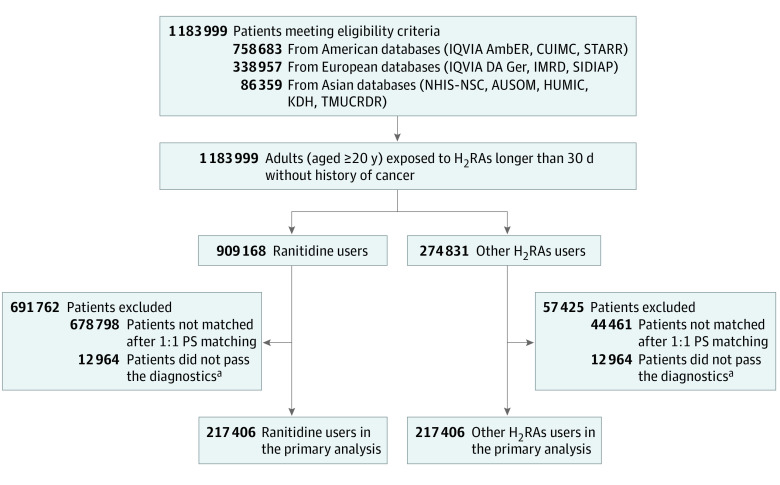
Figure 1. Patient Selection Flowchart.
AmbEMR indicates IQVIA US Ambulatory Electronic Medical Research; AUSOM, Ajou University School of Medicine; H2RA, histamine-2 receptor antagonist; CUIMC, Columbia University Irving Medical Center data warehouse; HUMIC, Hanyang University Medical Center; IMRD, UK’s IQVIA Medical Research Data; IQVIA DA Ger, IQVIA Disease Analyzer Germany; KDH, Kandong Sacred Heart Hospital; NHIS-NSC, Korean National Health Insurance System-National Sample Cohort; SIDIAP, The Information System for Research in Primary Care; STARR, Stanford University database warehouse; TMUCRD, Taipei Medical University Clinical Research Database.
aDiagnostic criteria indicate satisfaction of both empirical equipoise and balance after 1:1 propensity score (PS) matching; the study diagnostic criteria included empirical equipoise and sufficient balance of covariates after PS adjustment, and the empirical equipoise was satisfied when the preference scores of most patients (a transformation of the propensity score adjusting for different prevalence of treatments) in both groups were between 0.3 and 0.7. The balance between the target and comparator cohorts was considered sufficient if the absolute standardized mean difference of all covariates was not greater than 0.1.