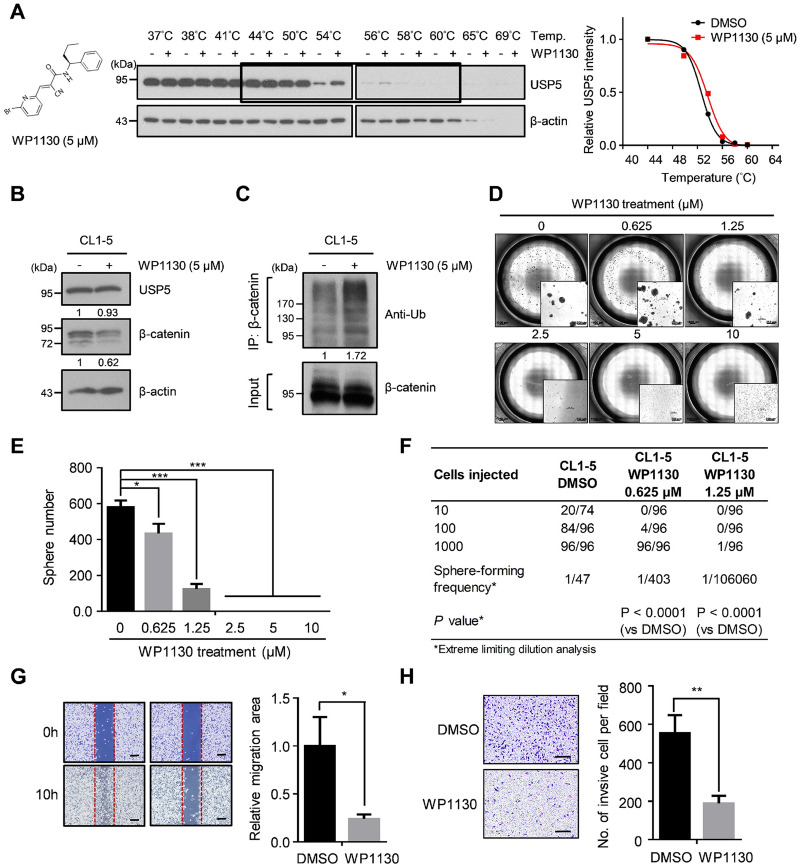
Fig. 5.
Targeting USP5 with WP1130 suppresses β-catenin expression, stemness and cell motility. A CETSA-based determination of interaction between WP1130 and USP5. Chemical structure of WP1130 (left). The results of immunoblotting of USP5 thermal aggregation curves of WP1130 at 5µM compared to DMSO control sample (middle). β-actin was used as an internal control. Representative images are shown. The band intensities of USP5 were normalized with respect to the intensity at 44 °C (right). B The protein levels of USP5 and β-catenin in CL1-5 cells treated with either DMSO (control) or 5 µM WP1130. β-actin was used as an internal control. C DMSO- or WP1130-treated CL1-5 cells were treated with 10 µM MG132 for 6 h and then subjected to immunoprecipitation under denaturing conditions using anti-β-catenin antibodies. The ubiquitination of β-catenin was detected by western blotting using an anti-ubiquitin antibody. D and E DMSO- and WP1130-treated CL1-5 cells were cultured at 10,000 cells per well in low-attachment plates to assess sphere formation. Representative stitched brightfield images were produced using NIS-Elements software (Nikon) (D), and spheres were quantified after 14 days, **P < 0.01 by a two-tailed Student’s t test (E). F The frequency of sphere-initiating cells. DMSO- and WP1130-treated CL1-5 cells were plated in low-attachment 96-well plates for a limiting-dilution assay. The percentage of wells with spheres was measured. The frequency and P-value were calculated using ELDA software. G Wound healing analysis of DMSO- and WP1130-treated CL1-5 cells at 10 h. Scale bar: 200 μm. H Transwell invasion analysis of DMSO- and WP1130-treated CL1-5 cells. Scale bar: 200 μm. In all bar plots, P-values were determined by a two-tailed Student’s t test