Figure 3.
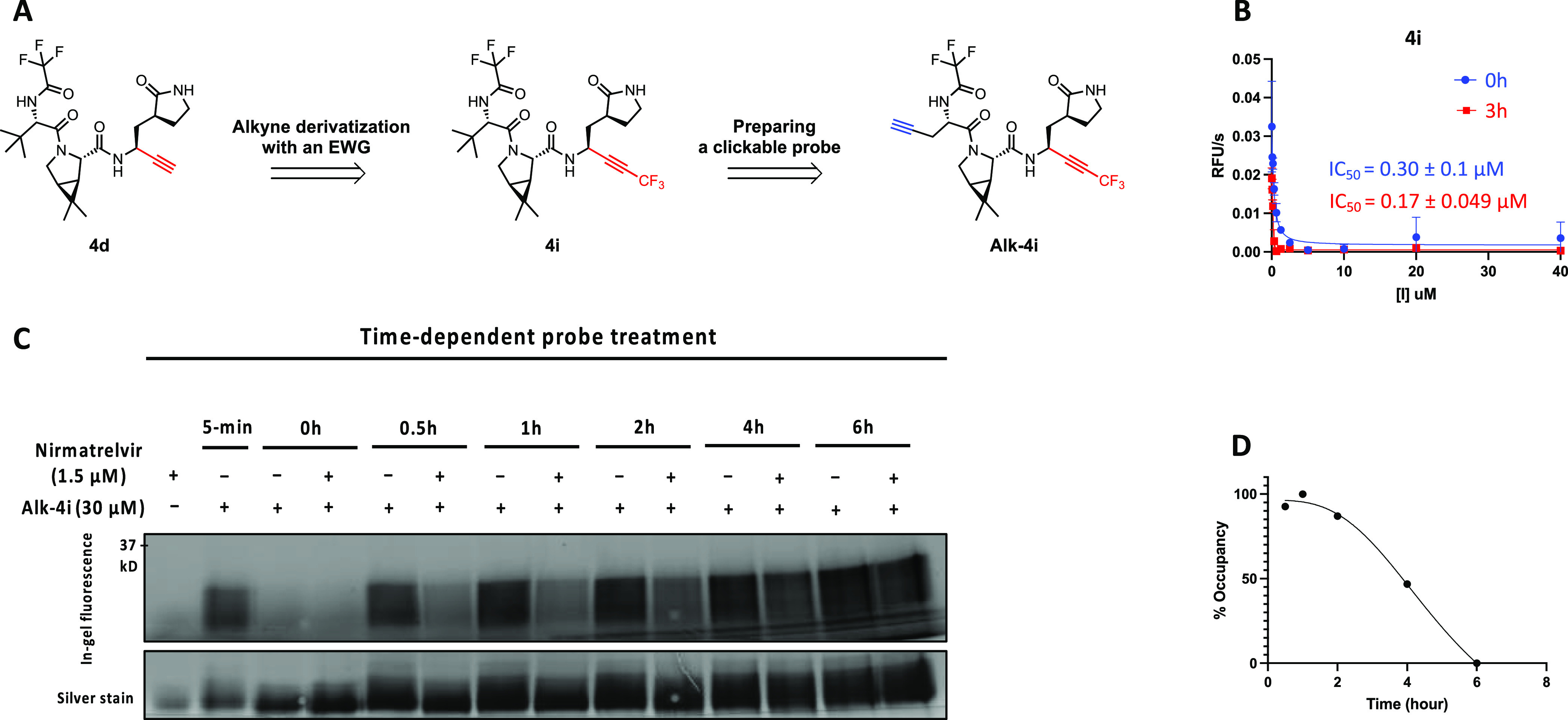
Tuning up alkyne reactivity through a chemical modification. (A) Alkyne substitution with an electron-withdrawing group (EWG), trifluoromethyl (CF3), to tune its warhead reactivity. A clickable probe, Alk-4i, was derived from 4i by replacing the solvent-exposed tert-butyl group with an alkyne reporter tag. (B) 4i inhibited Mpro and dramatically increased the rate of Mpro inactivation in the FRET-based enzymatic assay. (C) Measurements of the residence time of nirmatrelvir (4g) with Mpro by using Alk-4i in a competitive labeling experiment. Treatment of Mpro with a saturating concentration of nirmatrelvir (1.5 μM) for 30 min, followed by treatment with an excessive concentration (30 μM) of Alk-4i, revealed the dissociation kinetics of nirmatrelvir from Mpro. (D) Change of Mpro occupancy by nirmatrelvir over time. The data in (C) were quantified and normalized to generate the temporal curve.
