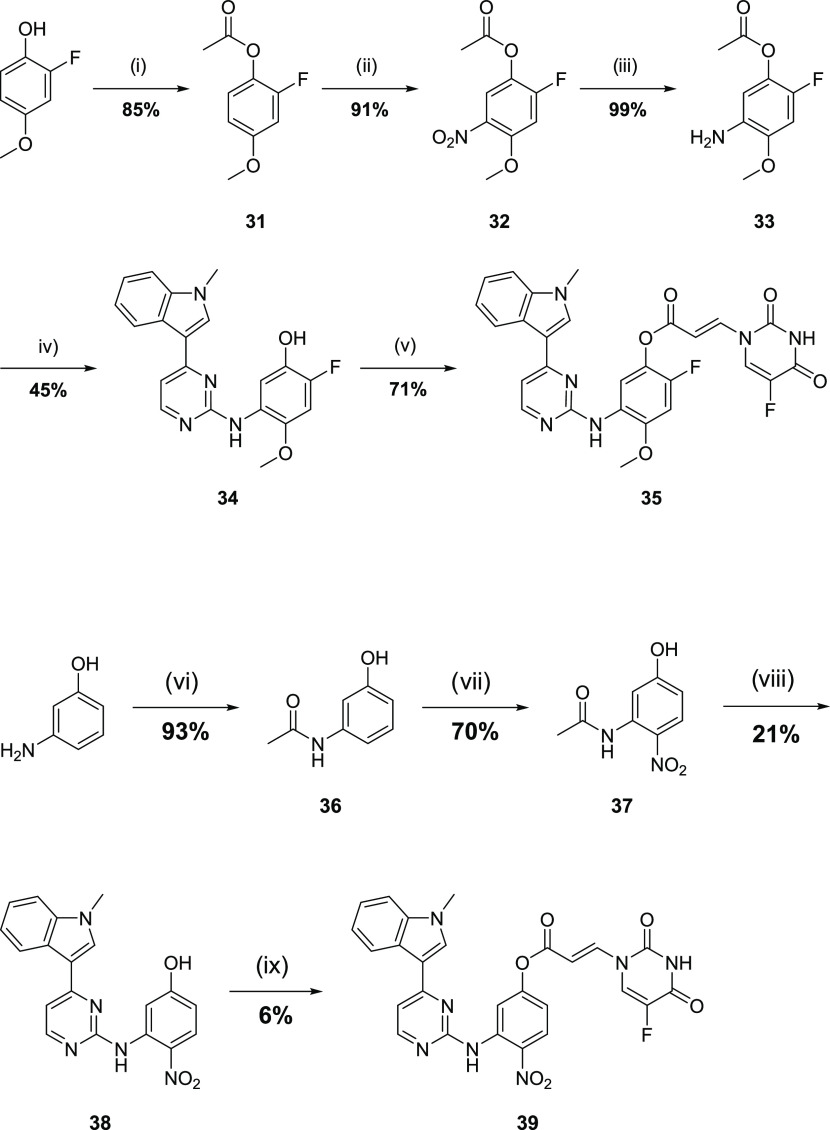
Scheme 3. Synthetic Route for the Formation of the Anilinopyrimidine Acrylates.
Reagents and conditions: (i) acetyl chloride, Et3N, DCM, rt, 18 h; (ii) HNO3, H2SO4, AcOH, 0 °C; 1 h, (iii) H2, Pd/C, MeOH, rt, 24 h; (iv) 3-(2-chloropyrimidin-4-yl)-1-methyl-1H-indole,18p-TSA, 2-pentanol, 110 °C, 18 h; (v) 16, DCC, DMAP, DMF, 0 °C—rt, 5 h; (vi) Ac2O, THF, rt, 1 h; (vii) KNO3, trifluoroacetic acid (TFA), 0 °C, 1 h; (viii) (a) 12 M HCl (aq), reflux, 2 h, (b) 3-(2-chloropyrimidin-4-yl)-1-methyl-1H-indole, p-TSA, 2-pentanol, 110 °C, 18 h; and (ix) 16, DCC, DMAP, DMF, rt, 18 h.