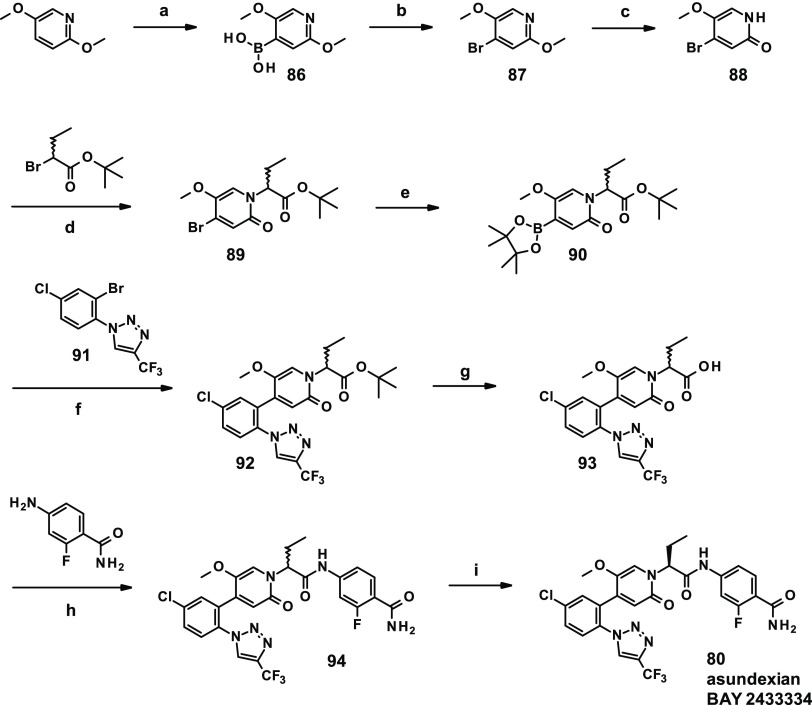
Scheme 1. Synthesis of Asundexian (BAY 2433334, 80).
Reagents and conditions: (a) (i) LDA, THF, (ii) B(Oi-Pr)3, −78 °C → RT, (iii) aq. HCl, 72%; (b) CuBr2, MeOH/H2O, 100 °C/microwave, 65%; (c) pyridine hydrobromide, DMF, 100 °C, 69%; (d) tert-butyl 2-bromobutanoate, K2CO3, DMF, 50 °C, 53%; (e) bis(pinacolato)diboron, Pd(dppf)Cl2-DCM complex, KOAc, 1,4-dioxane, 80 °C; (f) Pd(dppf)Cl2-DCM complex, K2CO3, 1,4-dioxane, 80 °C, 59% for two steps; (g) 4 M HCl/1,4-dioxane, RT, 99%; (h) T3P (50% in ethyl acetate), pyridine, 40 °C, 84%; (i) enantiomer separation.