Abstract
BACKGROUND: The societal costs of Alzheimer disease (AD) are considerable. Cost data stratified by cost category (direct and indirect) and AD severity in the United States are limited.
OBJECTIVE: To describe out-of-pocket (OOP) expenses and indirect costs from unpaid caregiving and work impairment among patients with AD by severity and among patients with mild cognitive impairment (MCI) in a representative sample of the US population.
METHODS: Data from the Health and Retirement Study (HRS) were used. HRS respondents were included if they reported an AD diagnosis or were considered as having MCI based on their cognitive performance. MCI and AD severity staging was performed using a crosswalk from results of the modified Telephone Interview of Cognitive Status to the Mini-Mental State Examination. OOP expenses were assessed along with indirect costs (costs to caregivers from providing unpaid help and costs to employers). Sensitivity analyses were performed by varying assumptions of caregiver employment, missed workdays, and early retirement. Patients with AD were stratified by nursing home status, type of insurance, and income level. All cost calculations applied sampling weights.
RESULTS: A total of 18,786 patients were analyzed. Patients with MCI (n = 17,885) and AD (n = 901) were aged 67.8 ± 10.7 and 80.9 ± 9.3 years, were 55.7% and 63.3% female, and were 28.3% and 0.9% employed, respectively. OOP expenses per patient per month increased with AD severity, ranging from $420 in mild to $903 in severe AD but were higher in MCI ($554) than in mild AD. Indirect costs to employers were similar across the AD continuum ($197-$242). Costs from unpaid caregiving generally increased by disease severity, from $72 (MCI) to $1,298 (severe AD). Total OOP and indirect costs increased by disease severity, from $869 (MCI) to $2,398 (severe AD). Sensitivity analysis assuming nonworking caregivers and zero costs to employers decreased the total OOP and indirect costs by 32%-53%. OOP expenses were higher for patients with AD who had private insurance (P < 0.01), had higher incomes (P < 0.01), or were in nursing homes (P < 0.01). Indirect costs to caregivers were lower for patients with AD in nursing homes ($600 vs $1,372, P < 0.01). Total indirect costs were higher for patients with AD with lower incomes ($1,498 vs $1,136, P < 0.01) and for those not in nursing homes ($1,571 vs $799, P < 0.01).
CONCLUSIONS: This study shows that OOP expenses and indirect costs increase with AD severity, OOP expenses increase with higher income, subscription of private insurance, and nursing home residency, and total indirect costs decrease with higher income and nursing home residency in the United States.
DISCLOSURES This study was financially sponsored by Eisai. Drs Zhang and Tahami are employees of Eisai. Drs Chandak, Khachatryan, and Hummel are employees of Certara; Certara is a paid consultant to Eisai. The views expressed here are those of the authors and are not to be attributed to their respective affiliations. Laura De Benedetti, BSc, provided medical writing support to the manuscript; she is an employee of Certara.
Plain language summary
We measured the costs of patients with Alzheimer disease (AD) or with mild cognitive impairment (MCI) in the United States. Out-of-pocket (OOP) expenses ranged between $420 and $903 per patient per month (PPPM), depending on disease severity. Indirect costs to employers were similar across disease severities. Indirect costs to caregivers ranged from $72 to $1,298 PPPM. The total OOP and indirect cost of MCI and AD ranged from $869 to $2,398 and increased with disease severity.
Implications for managed care pharmacy
The study examines the impact of AD severity on patient OOP expenses and indirect costs in the United States considering factors such as insurance, income, and nursing home status. Results highlight the need for early detection and effective interventions to reduce financial burden and socioeconomic disparities. Higher income results in increased OOP expenses, whereas nursing home residency lowers indirect costs. These findings indicate potential disparities in health care access and use.
Alzheimer disease (AD) is a chronic progressive neurodegenerative disease affecting more than 6.5 million Americans older than 65 years.1 This number is projected to more than double by 2060.1,2 As the prevalence of AD increases, so do the societal costs on patients, caregivers, and society.3-5
The costs of a disease can be categorized into direct, indirect, and intangible costs. Direct costs are health care and services provided where money is explicitly exchanged, including medical costs and nonmedical costs (social care costs). Indirect costs are resources lost or invested where no money is exchanged, including lost productivity for patients and caregivers and the cost of informal care.6-8 Intangible costs are nonmaterial costs better expressed in qualitative terms, which are not typically covered in economic analyses, including the impact of the disease on the quality of life (QoL) of the patients and caregivers.8 An overview of the cost categories and examples in AD is provided in Supplementary Table 1 (available in online article).
The economic burden of AD is considerable. For 2022, the total direct costs for patients with AD and other dementia in the United States are projected to be $321 billion (2022 US dollars), including $206 billion of Medicare and Medicaid payments (64%) and $81 billion out-of-pocket (OOP) expenses borne by the patients and their families (25%).5 In 2021, an estimated 16 billion hours were spent by unpaid caregivers of patients with dementia in the United States, which is valued at around $272 billion (2021 US dollars).5 Thus, a large proportion of direct and indirect costs for patients with AD and dementia are borne by the patients, their families, and their friends (OOP expenses and unpaid caregiving).
Intangible costs associated with AD are difficult to measure. There is wide variation in patients’ QoL across studies, which may be explained by patients’ altered cognition or capacity to assess the situation.6,8 However, it is clear when patient QoL is evaluated by caregivers that disease severity negatively impacts patient and caregiver QoL.9-13
AD severity is a key driver of economic burden, with direct and indirect costs generally increasing with disease severity.6 Cost data stratified by cost category and severity in the United States are limited.14,15 A greater understanding of this breakdown in the United States can shed light on how costs progress during the course of AD, the most costly categories in need of solutions, and the benefit of AD treatments that slow down progression to more severe disease.
The objective of this study is to describe the OOP expenses and indirect costs among patients with AD by severity and among patients with mild cognitive impairment (MCI) in the United States.
Methods
DATA SOURCE
The Health and Retirement Study (HRS) provides a representative sample of the US population containing data for Americans aged older than 50 years.16-18 Comprehensive interviews are conducted bi-annually, capturing relevant aspects of direct and indirect costs incurred by the respondents. A new cohort of individuals aged 51-56 is added every 6 years, leading to more than 43,000 individuals interviewed to date.18 The HRS is supported by the National Institute on Aging and the Social Security Administration. More details on the HRS can be found elsewhere.17,18
For this study, HRS data were downloaded on March 16, 2021. At the time of access, the latest available data were current as of December 31, 2018. Three main data sources within the HRS were used for this study: the HRS Core and Exit databases (1994-2018); the RAND HRS Longitudinal File (1994-2018); and the Aging, Demographics, and Memory Study (ADAMS) data (2001-2009).19-24 The HRS Core and Exit databases are a national longitudinal study of the economic, health, marital, and family status, as well as the public and private support systems, of older Americans. The RAND HRS Longitudinal File is a cleaned and streamlined data product containing public information (ie, no restricted data) from the HRS Core and Exit interview. ADAMS data are from 1,770 HRS respondents, aged 70 years or older, selected from the HRS 2000 and 2002 waves based on the score on the self- or proxy-cognitive assessment measure.
STUDY POPULATION
HRS respondents were included in this study if they reported an AD diagnosis (patients with AD) or if they were considered as having MCI based on their cognitive performance (patients with MCI). The Mini-Mental State Examination (MMSE) can be used to identify patients with MCI,22,23 and to categorize patients with AD into mild, moderate, or severe disease categories.24,25 The MMSE correlates well with the modified Telephone Interview of Cognitive Status (TICS-m), which is part of the bi-annual interviews of HRS.26 The TICS-m questions are provided in Supplementary Table 2. The TICS-m has been validated to identify those with and without dementia with satisfying sensitivity (83.3%) and specificity (81.6%).27
Because cutoffs do not exist for the TICS-m to categorize the HRS respondents into MCI or mild, moderate, and severe AD, a crosswalk to MMSE applying equipercentile equating was performed using ADAMS data.28,29 The latter is a substudy of HRS to gather in-depth information on the cognitive status of the respondents, and, as such, it collects both the TICS-m and MMSE of the respondents. Considering that cutoffs may vary by education levels, the TICS-m collected in HRS was adjusted for education.30 Further information on the crosswalk and the education adjustment can be found in Supplementary Exhibit 1.
The MMSE cutoffs and their corresponding education-adjusted TICS-m cutoffs from the crosswalk that were used for MCI and AD severity categorization can be found in Table 1.
TABLE 1.
MCI and Alzheimer Disease Severity Categorization and Crosswalk From MMSE to the Education-Adjusted TICS-m
| Severity stage | MMSE score | Adjusted TICS-m score |
|---|---|---|
| Severe | 0-10 | 0-9 |
| Moderate | 11-20 | 10-16 |
| Mild | 21-24 | 17-19 |
| MCI | 25-27 | 20-22 |
| Normal | 28-30 | 23-35 |
MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination; TICS-m = modified Telephone Interview of Cognitive Status.
Note that respondents reporting an AD diagnosis who had an education-adjusted TICS-m greater than or equal to 17 (ie, considered as mild, MCI, or normal based on their TICS-m) were assigned to patients with mild AD.
STUDY MEASURES
Study population characteristics were examined at the first visit, at which the respondents reported an AD diagnosis (for patients with AD) or at which the education-adjusted TICS-m was between 20 and 22 (for patients with MCI).
Costs reflect the 2 years prior to the first visit of AD diagnosis (for patients with AD) or of education-adjusted TICS-m was between 20 and 22 (for patients with MCI), as questions related to costs were generally asked for the previous 2 years’ time frame.
OOP expenses measured in the HRS included expenses for hospital, nursing home, outpatient surgery, doctor visits, dentist visits, medication, in-home medical care, and other health services.
Indirect costs included costs to caregivers from providing unpaid help calculated using the opportunity cost method.31 We assigned the value of lost productivity time for working caregivers and the value of lost leisure time for nonworking caregivers.32 Indirect costs also included costs to employers through absenteeism and replacement of patients and caregivers. More information on cost calculations, the HRS questions, and the assumptions used can be found in Supplementary Table 3.
The following sensitivity analyses were conducted to address the assumptions made when data were missing (employment, missed workdays, and early retirement information) for caregivers who were not spouses of respondents. The conservative scenario was as follows: for the indirect costs to caregivers, it was assumed the caregiver was not working; and for the indirect costs to employers, it was assumed the caregiver had zero missed workdays or early retirements. In the nonconservative scenario, it was assumed the caregiver was working when calculating indirect costs to caregivers.
Costs were generally calculated per patient per month (PPPM) and were stratified by MCI and AD severity. In addition, the OOP expenses and indirect costs of patients with AD were stratified by insurance type (patients with and without private insurance), nursing home status at baseline (patients living in a nursing home vs not living in a nursing home), and income level, the latter being categorized into poor (income < 125% of the federal poverty level [FPL]), low (between 125% and 199% of the FPL), middle (between 200% and 399% of the FPL), and high income (>399% of the FPL), respectively.33
STATISTICAL ANALYSES
For all descriptive analyses, categorical variables were summarized by number of observations and percentage. Continuous variables were summarized using descriptive statistics (n, mean with SD or median with interquartile range). Each variable was described with the frequencies of missing (eg, because of a part of the question/interview missing) and “not known” values (ie, when respondents replied that something was unknown).
In case subitems of the TICS-m were missing, we performed a multivariate, regression-based imputation, which has been described previously,34 using a combination of relevant demographic, health, and economic variables as well cognitive variables from prior and current surveys to have as much cognitive information as possible on the respondents.
If the TICS-m was not available at baseline for respondents diagnosed with AD, the TICS-m measurement of 1 or maximum 2 visits before or after (whichever was closest) the visit indicating AD diagnosis was used as the baseline TICS-m measurement. Otherwise, the baseline TICS-m was set to “missing.”
An adjustment of the costs to 2021 US dollars was conducted using the corresponding inflation rate from the medical care component of the US Consumer Price Index.35
All cost calculations applied sampling weights developed by the HRS to generate nationally representative results. Costs were presented as mean (SD), 95% CI, and median (interquartile range). P values for cost comparisons were provided by applying a t-test. No multiplicity adjustment was performed.
All statistical analyses were conducted using SAS (version 9.4 TS1M4; SAS Institute, Inc.).
Results
STUDY POPULATION CHARACTERIZATION
A flowchart for the selection of patients into this study is available in Supplementary Figure 1. From 42,233 HRS survey respondents assessed, 18,786 met the inclusion criteria of reporting an AD diagnosis or meeting the TICS-m cutoff for MCI (TICS-m between 20 and 22, see the subsection “Study Population” in the Methods section). Thus, 18,786 respondents were finally included in the analysis: 17,885 with MCI, and 901 reporting AD, corresponding to a prevalence of 43.0% and 3.2% for MCI and AD, respectively.
The years of assessment of study population characteristics and costs are detailed in Supplementary Table 4. Patients with MCI were identified from 1994 to 2008, however AD diagnosis was only asked in the surveys from 2010 onwards. Therefore, patients with AD could only be included in this study starting in the year 2010.
The demographic characteristics of the study population by MCI and AD severities are shown in Table 2. Patients with less severe disease were younger, had a higher income, and showed a higher proportion of being employed. The proportion of patients with high school or general educational diploma was similar across MCI and AD severities, and very few patients reported missed workdays or early retirement. The proportion of patients living in a nursing home increased with disease severity, except for patients with severe AD who showed a lower percentage than patients with mild and moderate AD (22.7% vs 26.3% and 30.5% for patients with severe AD vs patients with mild and moderate AD, respectively). The annual income from individual earnings decreased with severity and was highest in patients with MCI ($11,077) and lowest in patients with severe AD ($0).
TABLE 2.
Demographic Characteristics, Clinical and Lifestyle Characteristics, and Characteristics of Caregiving of the Study Population by MCI and AD Severities
| Patients with MCI (n = 17,885) | Patients with mild ADa (n = 171) | Patients with moderate AD (n = 262) | Patients with severe AD (n = 176) | Total patients with ADb (n = 901) | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, mean (SD), years | 67.8 (10.7) | 78.7 (9.0) | 81.1 (8.0) | 82.0 (7.9) | 80.9 (9.3) |
| Female sex, n (%) | 9,955 (55.7) | 97 (56.7) | 152 (58.0) | 113 (64.2) | 570 (63.3) |
| Hispanic ethnicity, n (%) | 1,919 (10.7) | 30 (17.5) | 31 (11.9) | 17 (9.7) | 119 (13.2) |
| Highest level of education, n (%) | |||||
| Less than high school | 4,444 (24.8) | 65 (38.0) | 89 (34.0) | 54 (30.7) | 322 (35.7) |
| High school/GED | 9,197 (51.4) | 80 (46.8) | 123 (46.9) | 92 (52.3) | 428 (47.5) |
| Some college or higher | 4,244 (23.7) | 26 (15.2) | 50 (19.1) | 33 (18.8) | 151 (16.8) |
| Marital status, n (%) | |||||
| Married or partnered | 11,348 (63.4) | 66 (38.6) | 108 (41.2) | 71 (40.3) | 300 (33.3) |
| Married, spouse absent | 135 (0.8) | 10 (5.8) | 18 (6.9) | 8 (4.5) | 58 (6.4) |
| Separated/divorced | 2,098 (11.7) | 25 (14.6) | 21 (8.0) | 18 (10.2) | 94 (10.4) |
| Widowed | 3,591 (20.1) | 65 (38.0) | 108 (41.2) | 75 (42.6) | 421 (46.7) |
| Never married | 712 (4.0) | 5 (2.9) | 7 (2.7) | 4 (2.3) | 28 (3.1) |
| Employment, n (%) | |||||
| Working for pay (employed) | 4,987 (27.8) | 4 (2.3) | 0 (0.0) | 1 (0.6) | 8 (0.9) |
| Not working for pay (unemployed, disabled, retired etc) | 12,642 (70.7) | 167 (97.7) | 261 (99.6) | 175 (99.4) | 892 (99.0) |
| Missing | 256 (1.4) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.1) |
| Annual income, mean (SD), USD | 11,077 (33,887) | 1,210.5 (8,213.5) | 157.4 (2,177.3) | 0.0 (0.0) | 385.9 (4,158.6) |
| Early retirement, n (%) | 7 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of workdays missed per month, mean (SD) | 0.1 (0.9) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.3) |
| Currently living in nursing home, n (%) | 96 (0.5) | 45 (26.3) | 80 (30.5) | 40 (22.7) | 322 (35.7) |
| Clinical and lifestyle characteristics | |||||
| Chronic conditions, n (%)c | |||||
| High blood pressure | 9,319 (52.1) | 135 (78.9) | 194 (74.0) | 122 (69.3) | 682 (75.7) |
| Diabetes | 3,350 (18.7) | 59 (34.) | 85 (32.4) | 65 (36.9) | 290 (32.2) |
| Cancer | 2,301 (12.9) | 42 (24.6) | 57 (21.8) | 47 (26.7) | 209 (23.2) |
| Lung disease | 1,531 (8.6) | 46 (26.9) | 37 (14.1) | 21 (11.9) | 148 (16.4) |
| Heart problems | 4,115 (23.0) | 93 (54.4) | 113 (43.1) | 70 (39.8) | 385 (42.7) |
| Stroke | 1,341 (7.5) | 56 (32.7) | 72 (27.5) | 42 (23.9) | 285 (31.6) |
| Emotional, nervous, or psychiatric problems | 2,175 (12.2) | 72 (42.1) | 101 (38.5) | 51 (29.0) | 376 (41.7) |
| Depression | 1,300 (7.3) | 82 (48.0) | 106 (40.5) | 52 (29.5) | 377 (41.8) |
| Patient-reported medication use for, n (%)c | |||||
| High blood pressure | 9,086 (50.8) | 125 (73.1) | 176 (67.2) | 106 (60.2) | 587 (65.1) |
| Diabetes | 2,640 (14.8) | 44 (25.7) | 55 (21.0) | 44 (25.0) | 198 (22.0) |
| Cancer | 700 (3.9) | 11 (6.4) | 13 (5.0) | 9 (5.1) | 46 (5.1) |
| Lung disease | 1,226 (6.9) | 33 (19.3) | 23 (8.8) | 12 (6.8) | 94 (10.4) |
| Heart problems | 3,123 (17.5) | 75 (43.9) | 88 (33.6) | 47 (26.7) | 269 (29.9) |
| Stroke | 697 (3.9) | 32 (18.7) | 38 (14.5) | 24 (13.6) | 140 (15.5) |
| Psychiatric problems | 883 (4.9) | 36 (21.1) | 45 (17.2) | 27 (15.3) | 172 (19.1) |
| Presence of any chronic condition or patient-reported medication use | 1,3021 (72.8) | 161 (94.2) | 248 (94.) | 158 (89.8) | 849 (94.2) |
| CESD score, mean (SD) | 1.6 (2.0) | 2.8 (2.5) | 1.9 (2.2) | 1.8 (2.2) | 2.2 (2.4) |
| Smoking status: never smoked, n (%) | 7,609 (42.6) | 81 (48.2) | 125 (47.9) | 87 (49.4) | 442 (49.4) |
| Alcohol consumption, n (%) | |||||
| < 1 day | 12,121 (67.8) | 137 (81.5) | 230 (87.8) | 156 (89.1) | 798 (89.0) |
| 1-2 days | 3,097 (17.3) | 15 (8.9) | 16 (6.1) | 12 (6.9) | 53 (5.9) |
| ≥3 days | 2,662 (14.9) | 16 (9.4) | 16 (6.1) | 7 (4.0) | 46 (5.1) |
| Having difficulties with any ADL/IADL, n (%) | 4,037 (22.6) | 139 (81.3) | 232 (88.5) | 164 (93.2) | 819 (90.9) |
| Mapped BADLS score, mean (SD) | 1.7 (3.8) | 12.9 (9.4) | 14.5 (8.5) | 17.3 (7.5) | 16.6 (8.6) |
| Characteristics of caregiving | |||||
| Caregiver needed, n (%) | 1,553 (11.8) | 121 (70.8) | 206 (78.6) | 156 (88.6) | 735 (81.6) |
| If caregiver needed, relationship with patient, n (%) | n = 1,553d | n = 121d | n = 206d | n = 156d | n = 735d |
| Spouse | 458 (29.5) | 21 (17.4) | 45 (21.8) | 45 (48.8) | 158 (21.5) |
| Son/daughter | 422 (27.2) | 61 (50.4) | 86 (41.7) | 100 (64.1) | 421 (57.3) |
| Spouse/partner of son/daughter | 48 (3.1) | 10 (8.3) | 14 (6.8) | 18 (11.5) | 82 (11.2) |
| Grandchild | 97 (6.2) | 15 (12.4) | 14 (6.8) | 23 (14.7) | 90 (12.2) |
| Other relative | 98 (6.3) | 1 (0.8) | 15 (7.3) | 14 (9.0) | 77 (10.5) |
| Other individual | 149 (9.6) | 17 (14.0) | 17 (8.3) | 20 (12.8) | 101 (13.7) |
| Organization/paid caregiver | 186 (12.0) | 58 (47.9) | 122 (59.2) | 105 (67.3) | 479 (65.2) |
| Total hours of unpaid help in the last month, mean (SD) | 7.7 (52.7) | 109.7 (197.5) | 102.3 (185.8) | 181.5 (238.5) | 173.6 (229.4) |
| If caregiver needed, employment of caregiver, n (%) | n = 1,553d | n = 121d | n = 206d | n = 156d | n = 735d |
| Working for pay (employed) | 119 (7.7) | 2 (1.7) | 3 (1.5) | 4 (2.6) | 13 (1.8) |
| Not working for pay (unemployed, disabled, retired etc.) | 339 (21.8) | 19 (15.7) | 42 (20.4) | 41 (26.3) | 145 (19.7) |
| Not known (if caregiver was not the spouse) | 1,095 (70.5) | 100 (82.6) | 161 (78.2.5) | 111 (71.2) | 577 (78.5) |
| Annual income of caregiver, mean (SD), USD | 12,615 (34,065) | 2,693.4 (12,005) | 4,017.5 (15,316) | 3,177.2 (19,974) | 3,841.7 (21,600) |
aPatients with mild AD include patients with AD with TICS-m greater than or equal to 17, ie, considered as mild, MCI, or normal based on their TICS-m.
bTotal patients with AD include patients with AD with missing TICS-m (n = 292).
cNonexclusive categories.
dDenominator was the number of respondents who needed a caregiver.
AD = Alzheimer disease; ADL = activities of daily living; BADLS = Bristol activities of daily living score (higher scores indicating more impaired ADL); CESD = Center for Epidemiologic Studies Depression (higher scores indicated a higher frequency of depressive symptoms); GED = general educational diploma; IADL = instrumental activities of daily living; MCI = mild cognitive impairment; TICS-m = modified Telephone Interview of Cognitive Status.
The clinical and lifestyle characteristics of the study population are shown in Table 2. Chronic conditions or medication use and impairment of activities of daily living (ADL) or instrumental ADL increased with disease severity, with an increase in the proportion of patients having difficulties with any ADL/instrumental ADL and an increase in the mapped Bristol ADL score.36 The Center for Epidemiologic Studies Depression37 score was similar across disease severities, with the exception being that patients with mild AD showed a higher Center for Epidemiologic Studies Depression score than the other disease severities, indicating that patients with mild AD experience more depression than those with MCI or moderate and severe AD. This was also supported by the proportion of patients reporting depression as a chronic condition, which was highest among patients with mild AD (48.0%).
The characteristics of caregiving are shown in Table 2. The proportion of patients requiring a caregiver increased with disease severity. In most cases, the spouse or the children cared for the patient. Formal caregiving and the hours of unpaid help provided to the patient increased with disease severity. The percentage of caregivers who worked for pay was low (between 0.7% for patients with MCI and 2.3% for patients with severe AD), but the percentage of missing employment information was high, except for MCI (58.5%-63.1% for patients with AD vs 6.1% for patients with MCI). The annual income of caregivers was highest for caregivers caring for patients with MCI ($12,615.00) and lowest for caregivers caring for patients with mild AD ($2,693.40) and showed a large variability (SD≤34,065).
The health resource use is shown in Supplementary Table 5. Patients with less severe disease generally reported less health resource use (eg, hospital admission, use of home health service and special health facility, and admission to and number of nights spent in a nursing home).
COSTS
The average OOP costs PPPM are shown in Figure 1. OOP costs increased with AD severity, ranging from $420 PPPM in patients with mild AD to $903 PPPM in patients with severe AD, but OOP expenses were higher in patients with MCI ($554) as compared with patients with mild AD.
FIGURE 1.
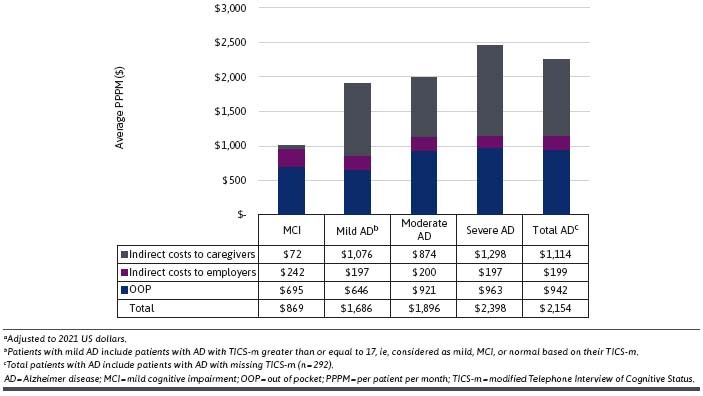
OOP Expenses, Indirect Costs, and Total Costs, Average PPPM Costs in USDa
The indirect costs for patients with MCI and AD are also shown in Figure 1. The indirect costs to employers were similar for patients with MCI and AD ($197-$242 PPPM). The indirect costs to caregivers from providing unpaid help generally increased by disease severity, from $72 PPPM for patients with MCI to $1,298 PPPM for patients with severe AD but were slightly higher in patients with mild AD as compared with patients with moderate AD ($1,011 and $874 PPPM, respectively).
Similar trends were found for median costs PPPM, ie, generally increasing costs by disease severity (Supplementary Figure 2).
The total costs for patients with MCI and AD are shown at the bottom of Figure 1. The total costs were calculated as the sum of the OOP expenses and indirect costs. Total costs PPPM increased by disease severity and ranged from $869 (MCI) to $2,398 (severe AD). Mean, 95% CI, and median OOP expenses, indirect costs to caregivers, indirect costs to employers, and total costs are shown in Supplementary Table 6.
The results of the sensitivity analyses varying the assumptions on caregiver employment, missed workdays, and early retirement, in case that information was missing, are presented in Supplementary Figure 3. In the conservative scenario (assuming the caregiver was not working, had zero missed workdays, and had no early retirement), the indirect costs to employers were negligible for the patients with AD, as information on caregiver employment, missed workdays, and early retirement was missing for 58.5%-63.1% of the caregivers and patients were rarely employed at that stage. For patients with MCI, the indirect costs to employers amounted to $45 PPPM under the conservative scenario. The indirect costs to caregivers also decreased under the conservative scenario in which it was assumed that the caregiver was not working (eg, from $874 to $611 PPPM in patients with moderate AD). Total costs in the conservative scenario decreased by 32.0%-53.0% as compared with the main analysis. In the nonconservative scenario (assuming working caregivers when this information was missing), indirect costs to caregivers increased as compared with the main analysis (eg, from $874 to $1,184 PPPM in patients with moderate AD), resulting in total costs that were 14.1%-21.0% higher in patients with AD but the same results for patients with MCI as compared with the main analysis, of which the latter can be explained by a low percentage of missingness in caregiver employment (6.1% only) and thus less variation in the results.
Similar results were found for median costs PPPM (Supplementary Figure 4).
Stratified OOP costs are shown in Table 3. OOP costs were higher in privately insured patients with AD than in patients with AD without private insurance (P < 0.0001). OOP expenses generally increased with the level of income, and OOP expenses for patients with AD in the high-income category more than quadrupled as compared with poor patients with AD (P < 0.0001). OOP costs for patients with AD in a nursing home was $1,480 vs $521 for those not in a nursing home at baseline (P < 0.0001).
TABLE 3.
Stratified Out-of-Pocket Expenses, Average per Patient per Month Costs in US Dollarsa
| Mild AD | Moderate AD | Severe AD | Total AD | P valueb | ||
|---|---|---|---|---|---|---|
| Private insurance | nc | 44 | 81 | 53 | 262 | |
| Mean (SD) | 710.72 (1,297.94) | 1,283.44 (2,708.59) | 1,281.25 (2,706.05) | 1,255.46 (2,541.5) | P < 0.0001 | |
| No private insurance | nc | 122 | 172 | 118 | 605 | |
| Mean (SD) | 312.51 (855.85) | 609.48 (1,393.22) | 752.43 (1,769.29) | 673.21 (1,620.03) | ||
| Poor | n | 61 | 68 | 64 | 302 | |
| Mean (SD) | 161.53 (314.48) | 325.47 (605.46) | 375.04 (1,488.18) | 326.52 (903.92) | P < 0.0001 (poor vs high income) | |
| Low income | n | 40 | 74 | 38 | 235 | |
| Mean (SD) | 567.33 (1,151.26) | 763.18 (1,412.94) | 1,426.71 (2,979.97) | 842.03 (1,757.25) | ||
| Middle income | n | 44 | 74 | 46 | 235 | |
| Mean (SD) | 451.97 (723.42) | 1,172.71 (2,784.58) | 1,426.71 (2,979.97) | 1,092.33 (2,280.09) | ||
| High income | n | 26 | 46 | 28 | 129 | |
| Mean (SD) | 676.23 (1,638.98) | 935.32 (1,865.5) | 750.06 (1,396.66) | 1,395.35 (2,735.89) | ||
| Staying in nursing home | n | 45 | 80 | 40 | 322 | |
| Mean (SD) | 772.6 (1,613.45) | 1,345.19 (2,395.09) | 1,538.08 (2,251.81) | 1,479.81 (2,599.98) | P < 0.0001 | |
| Not staying in nursing home | n | 126 | 182 | 136 | 579 | |
| Mean (SD) | 293.08 (599.73) | 584.7 (1,651.78) | 729.55 (2,021.25) | 520.86 (1,428.83) |
aAdjusted to 2021 US dollars.
bP values shown are from a t-test for the comparison of the total Alzheimer disease values.
cThe n’s of these 2 patient groups (private vs no private insurance) do not add up to the overall number of patients per disease severity because 34 respondent answers were missing.
AD = Alzheimer disease.
Stratified indirect costs are shown in Table 4. Indirect costs to caregivers were lower for patients with AD in a nursing home than for those not in a nursing home at baseline ($600 vs $1,372, P < 0.0001). Indirect costs to caregivers for patients with AD with lower income were higher than for patients with higher income ($1,138 for poor patients with AD vs $929 for patients with AD with high income, P = 0.0076). Indirect costs to employers did not differ by income, insurance type, or nursing home status at baseline. Total indirect costs, ie, indirect costs to caregivers plus indirect costs to employers, were lower for patients with AD in nursing homes as compared with patients with AD not in nursing homes at baseline ($799 vs $1,571, P < 0.0001), and the total indirect costs for poor patients with AD were higher than for patients with AD with a high income ($1,498 for poor patients with AD vs $1,136 for patients with AD with a high income, P = 0.0089). Similar results were obtained for the sensitivity analyses (Supplementary Tables 7 and 8).
TABLE 4.
Stratified Indirect Costs, Average Per Patient Per Month Costs in US Dollarsa
| Mild AD | Moderate AD | Severe AD | Total AD | P valuea | ||
|---|---|---|---|---|---|---|
| Indirect costs to caregivers | ||||||
| Private insurance | nc | 44 | 81 | 53 | 262 | |
| Mean (SD) | 790.6 (990.98) | 1,021.44 (1,456.49) | 1,346.44 (1,313.9) | 1,149.81 (1,331.95) | 0.6239 | |
| No private insurance | nc | 122 | 172 | 118 | 605 | |
| Mean (SD) | 1,118.95 (1,346.18) | 814.28 (1,126.77) | 1,283.47 (1,227.4) | 1,103.18 (1,291.86) | ||
| Poor | n | 61 | 68 | 64 | 302 | |
| Mean (SD) | 1,396.64 (1,438.76) | 1,105.94 (1,315.19) | 1,534.42 (1,439.8) | 1,137.68 (1,331.35) | 0.0076 (poor vs high income) | |
| Low income | n | 40 | 74 | 38 | 235 | |
| Mean (SD) | 798.04 (1,155.71) | 805.59 (1,151.32) | 1,185.09 (1,136.94) | 1,137.68 (1,331.35) | ||
| Middle income | n | 44 | 74 | 46 | 235 | |
| Mean (SD) | 925.78 (1,186.47) | 824.69 (1,204.52) | 1,131.34 (929.77) | 990.29 (1,146.82) | ||
| High income | n | 26 | 46 | 28 | 129 | |
| Mean (SD) | 671.33 (876.87) | 769.34 (1,316.98) | 1,261.75 (1,355.43) | 929.28 (1,252.47) | ||
| Staying in nursing home | n | 45 | 80 | 40 | 322 | |
| Mean (SD) | 662.74 (876.32) | 484.75 (824.81) | 941.6 (873.96) | 599.57 (837.46) | P < 0.0001 | |
| Not staying in nursing home | n | 126 | 182 | 136 | 579 | |
| Mean (SD) | 1,136.51 (1,348.91) | 1,050.62 (1,356.85) | 1,395.08 (1,323.25) | 1,371.77 (1,415.16) | ||
| Indirect costs to employers | ||||||
| Private insurance | nc | 44 | 81 | 53 | 262 | |
| Mean (SD) | 197.24 (0) | 197.24 (0) | 197.24 (0) | 199.6 (20.87) | 0.8926 | |
| No private insurance | nc | 122 | 172 | 118 | 605 | |
| Mean (SD) | 198.12 (7.96) | 200.86 (52.73) | 197.24 (0) | 199.35 (32.32) | ||
| Poor | n | 61 | 68 | 64 | 302 | |
| Mean (SD) | 197.24 (0) | 197.24 (0) | 197.24 (0) | 199.22 (23.98) | 0.2193 (poor vs high income) | |
| Low income | n | 40 | 74 | 38 | 235 | |
| Mean (SD) | 197.54 (1.92) | 197.24 (0) | 197.24 (0) | 197.29 (0.82) | ||
| Middle income | n | 44 | 74 | 46 | 235 | |
| Mean (SD) | 197.77 (5.12) | 197.24 (0) | 197.24 (0) | 197.34 (2.24) | ||
| High income | n | 26 | 46 | 28 | 129 | |
| Mean (SD) | 199.57 (14.21) | 210 (98.35) | 197.24 (0) | 206.22 (61.98) | ||
| Staying in nursing home | n | 45 | 80 | 40 | 322 | |
| Mean (SD) | 197.39 (1.34) | 204.43 (74.74) | 197.24 (0) | 199.16 (38.55) | 0.8990 | |
| Not staying in nursing home | n | 126 | 182 | 136 | 579 | |
| Mean (SD) | 198.04 (7.79) | 197.29 (1.15) | 197.24 (0) | 199.46 (21.99) | ||
| Total indirect costs | ||||||
| Private insurance | nc | 44 | 81 | 53 | 262 | |
| Mean (SD) | 987.84 (990.98) | 1,218.68 (1,456.49) | 1,543.68 (1,313.9) | 1,349.42 (1,333.24) | 0.6222 | |
| No private insurance | nc | 122 | 172 | 118 | 605 | |
| Mean (SD) | 1,317.07 (1,345.47) | 1,015.14 (1,129.89) | 1,480.71 (1,227.4) | 1,302.53 (1,291.91) | ||
| Poor | n | 61 | 68 | 64 | 302 | |
| Mean (SD) | 1,593.87 (1,438.76) | 1,303.18 (1,315.19) | 1,731.66 (1,439.8) | 1,498.02 (1,405.09) | 0.0089 (poor vs high income) | |
| Low income | n | 40 | 74 | 38 | 235 | |
| Mean (SD) | 995.59 (1,155.5) | 1,002.83 (1,151.32) | 1,382.33 (1,136.94) | 1,334.98 (1,331.31) | ||
| Middle income | n | 44 | 74 | 46 | 235 | |
| Mean (SD) | 1,123.54 (1,186.07) | 1,021.93 (1,204.52) | 1,328.58 (929.77) | 1,187.62 (1,146.74) | ||
| High income | n | 26 | 46 | 28 | 129 | |
| Mean (SD) | 870.9 (875.21) | 979.34 (1,326.72) | 1,458.99 (1,355.43) | 1,135.5 (1,258.15) | ||
| Staying in nursing home | n | 45 | 80 | 40 | 322 | |
| Mean (SD) | 860.12 (876.22) | 689.18 (836.18) | 1,138.83 (873.96) | 798.73 (840.16) | P < 0.0001 | |
| Not staying in nursing home | n | 126 | 182 | 136 | 579 | |
| Mean (SD) | 1,334.55 (1,348.26) | 1,247.91 (1,356.84) | 1,592.32 (1,323.25) | 1,571.23 (1,414.93) | ||
aAdjusted to 2021 US dollars.
bP values shown are from a t-test for the comparison of the total AD values.
cThe n’s of these 2 patient groups (private vs no private insurance) do not add up to the overall number of patients per disease severity because a few respondent answers were missing.
AD = Alzheimer disease.
Discussion
In this study, patients with MCI and AD from a representative sample of the US population, the HRS, were assessed to derive OOP expenses and indirect costs PPPM, stratified by disease severity.
The US National Institute on Aging has stated that the HRS is a representative sample of the American population.16,38 HRS participants are selected based on multistage area probability sampling involving geographical stratification and clustering and oversampling of certain demographics. Weighting is used to account for differential probability of selection and differential nonresponse.17,39 The HRS has been used as a representative population in several other AD and cognition-related studies,14,40-43 and applying sampling weights in the cost calculations yields results that are representative for the US population. Patient characteristics of this study are also similar to those of a modeling study by Rajan et al in 2021, based on the large Chronic Hypertension and Pregnancy study and 2020 US Census data, further substantiating the representativeness of the HRS data.1
AD diagnosis was self-reported by patients, and the cognitive instrument TICS-m was used to stratify the AD patients into mild, moderate, and severe AD categories. Patients with MCI were identified based on their TICS-m score. OOP medical expenses were reported by the patients, and indirect costs were calculated as the sum of absenteeism and replacement costs because of missed workdays and early retirement, respectively, and of costs due to unpaid caregiving time.
OOP expenses increased with AD severity. Indirect costs to employers were similar across MCI and AD severities. Costs from unpaid caregiving generally increased by disease severity. Total OOP and indirect costs increased by disease severity. OOP costs were higher in privately insured patients with AD or for patients with AD with higher income. OOP expenses were higher for patients with AD living in a nursing home. Indirect costs to caregivers were lower for patients with AD living in a nursing home and were higher for patients with lower income. Total indirect costs, ie, indirect costs to caregivers plus indirect costs to employers, were lower for patients with AD living in a nursing home at baseline and were higher for patients with AD with a lower income, but they did not differ by insurance status. Sensitivity analyses varying the assumption made to calculate indirect costs showed similar results.
We assessed OOP expenses for patients with MCI and AD, which represent the parts of the direct costs that are fully borne by the patients and their families. Most studies assessing direct costs of AD focus on Medicare and Medicaid costs only, but OOP expenses amounted to 24% of the total annual per person direct costs in 2021 and should therefore not be neglected.
The cost estimates from this study provide a cross-sectional evaluation of the costs within the 2 years before the AD diagnosis or the first time of observing cognitive impairment based on the TICS-m, ie, costs at or around diagnosis. Other economic studies average the costs incurred in the years following diagnosis, or even focus on the last years of life, therefore likely providing higher cost estimates than our study.5,15,44
This study is novel as it provides the cost breakdown of AD by disease severity for the total cost and for each cost category.
LIMITATIONS
There were several limitations of this study. First, the identification of patients with AD was based on selfreported diagnoses of AD; therefore, under-reporting is likely, especially in mild or moderate cases. This is also reflected in the AD prevalence found for the HRS (3.2%), which is lower than the US prevalence (10.7%).5 On the other hand, the prevalence of MCI found in the HRS (43.0%) is higher than what is typically reported for an aging US population (12%-18% of people aged ≥60 years are living with MCI).5 The main reason for the overestimation in this study is that the cutoffs (TICS-m between 20 and 22) could have led to some patients without MCI being assigned to MCI, as the MMSE, which the cutoffs are based on, is known as a good instrument to distinguish between AD severities but has limitations in detecting MCI.45 Generally, disease severity categorization was based on the crosswalk between TICS-m and MMSE, and MMSE cutoffs translated to TICS-m cutoffs. The validation of TICS-m cutoffs could not be performed because of limited data, which could have resulted in some patients being assigned to an inappropriate disease severity. For other versions of the TICS-m (with a maximum score of 30 or 40; the one deployed in HRS has a maximum score of 35), there is debate in the literature about the ability to differentiate between MCI and AD.46-48
Second, because of the design of the HRS, the exact dates of the diagnosis of AD were not known, and first-reported visits had to be used instead. Considering that there may be a time lag between the date of AD diagnosis and reported TICS-m values, the disease severity category may have been overestimated or underestimated for some patients, although that time lag was typically not expected to be longer than 2 years.
Third, for around 10% of patients, there were discrepancies between AD diagnosis and assigned TICS-m score, ie, their TICS-m score was suggestive of MCI or normal cognitive status despite being diagnosed with AD. To assign disease severity for these patients, the AD diagnosis took precedence over TICS-m testing results, and these patients were subsequently categorized as having mild AD.
Fourth, about 30% of patients with AD did not have a TICS-m, and thus they could not be assigned a disease severity category. Comparison of patients with AD without a TICS-m with those having a TICS-m did not reveal any significant differences between these 2 patient groups regarding population characteristics or costs.
Further, employment and work productivity information were missing for 58.5%-63.1% of caregivers of patients with AD and for 6.1% of caregivers of patients with MCI. Subsequently, assumptions were made in terms of proportion of employed caregivers in line with those reported in the literature.15 Those assumptions were assessed in sensitivity analyses, resulting in a range of values that reflect the uncertainty in the indirect cost estimations.
Conclusions
The study demonstrates that both OOP expenses and indirect costs increase with the disease severity across the AD continuum and are influenced by factors such as type of insurance, income, and nursing home status. The study also provides an in-depth analysis of indirect costs, including absenteeism and replacement costs for patients and their caregivers, and unpaid caregiving time. These cost estimates can be used to calculate the total lifetime cost of the disease while accounting for disease severity from MCI to more severe AD health states and inform cost-effectiveness analysis and health policy.
ACKNOWLEDGMENTS
The authors would like to thank Laura De Benedetti, BSc, and Kulvinder Katie Singh, PharmD, for providing medical writing support to this manuscript.
REFERENCES
- 1.Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers Dement. 2021;17(12):1966-75. doi:10.1002/alz.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. 2018;14(2):121-9. doi:10.1016/j.jalz.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wimo A, Guerchet M, Ali GC, et al. . The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement. 2017;13(1):1-7. doi:10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM. Societal and family lifetime cost of dementia: Implications for policy. J Am Geriatr Soc. 2017;65(10):2169-75. doi:10.1111/jgs.15043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4): 700-89. doi:10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 6.Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The humanistic and economic burden of Alzheimer’s disease. Neurol Ther. 2022;11(2):525-51. doi:10.1007/s40120-022-00335-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson M, Schnaider Beeri M. Cost of Alzheimer’s disease. Dialogues Clin Neurosci. 2000;2(2):157-61. doi:10.31887/DCNS.2000.2.2/mdavidson [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Hayek YH, Wiley RE, Khoury CP, et al. . Tip of the iceberg: Assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J Alzheimers Dis. 2019;70(2):323-41. doi:10.3233/JAD-190426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumann C, Alexopoulos P, Perneczky R. Determinants of self- and carer-rated quality of life and caregiver burden in Alzheimer disease. Int J Geriatr Psychiatry. 2019;34(10):1378-85. doi:10.1002/gps.5126 [DOI] [PubMed] [Google Scholar]
- 10.Hongisto K, Hallikainen I, Selander T, et al. . Quality of life in relation to neuropsychiatric symptoms in Alzheimer’s disease: 5-year prospective ALSOVA cohort study. Int J Geriatr Psychiatry. 2018;33(1):47-57. doi:10.1002/gps.4666 [DOI] [PubMed] [Google Scholar]
- 11.Froelich L, Llado A, Khandker RK, et al. . Quality of life and caregiver burden of Alzheimer’s disease among community dwelling patients in Europe: Variation by disease severity and progression. J Alzheimers Dis Rep. 2021;5(1):791-804. doi:10.3233/ADR-210025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashizawa T, Igarashi A, Sakata Y, et al. . Impact of the severity of Alzheimer’s disease on the quality of life, activities of daily living, and caregiving costs for institutionalized patients on anti-Alzheimer medications in Japan. J Alzheimers Dis. 2021;81(1):367-74. doi:10.3233/JAD-201514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery W, Goren A, Kahle-Wrobleski K, Nakamura T, Ueda K. Alzheimer’s disease severity and its association with patient and caregiver quality of life in Japan: results of a community-based survey. BMC Geriatr. 2018;18(1):141. doi:10.1186/s12877-018-0831-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ton TGN, DeLeire T, May SG, et al. . The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Dement. 2017;13(3):217-24. doi:10.1016/j.jalz.2016.08.009 [DOI] [PubMed] [Google Scholar]
- 15.Robinson RL, Rentz DM, Andrews JS, et al. . Costs of early stage Alzheimer’s disease in the United States: Crosssectional analysis of a prospective cohort study (GERAS-US)1. J Alzheimers Dis. 2020;75(2):437-50. doi:10.3233/JAD-191212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Health and Retirement Study. The Health and Retirement Study. University of Michigan Institute for Social Research. Accessed April 7, 2022. https://hrs.isr.umich.edu/about [Google Scholar]
- 17.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: The Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576-85. doi:10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher GG, Ryan LH. Overview of the Health and Retirement Study and introduction to the special issue. Work Aging Retire. 2018;4(1):1-9. doi:10.1093/workar/wax032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health and Retirement Study. Core and Exit databases (1994-2018). Public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Data downloaded on March 16, 2021. [Google Scholar]
- 20.Health and Retirement Study. ADAMS data (2001-2009). Public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). Data downloaded on March 16, 2021. [Google Scholar]
- 21.Health and Retirement Study. RAND HRS longitudinal file (1994-2018). Sensitive dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging; (grant number NIA U01AG009740). Data downloaded on March 16, 2021. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-98. doi:10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Qiu Q, Qian S, et al. . Determining appropriate screening tools and cutoffs for cognitive impairment in the Chinese elderly. Front Psychiatry. 2021;12:773281. doi:10.3389/fpsyt.2021.773281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X, Li F, Chen S, Jia J. Associated factors of total costs of Alzheimer’s disease: A cluster-randomized observational study in China. J Alzheimers Dis. 2019;69(3):795-806. doi:10.3233/JAD-190166 [DOI] [PubMed] [Google Scholar]
- 25.Zaudig M. A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD-10 and DSM-III-R criteria. Int Psychogeriatr. 1992;4 Suppl 2:203-19. doi:10.1017/s1041610292001273 [DOI] [PubMed] [Google Scholar]
- 26.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1(2):111-7. [Google Scholar]
- 27.Knopman DS, Roberts RO, Geda YE, et al. . Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34-42. doi:10.1159/000255464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fong TG, Fearing MA, Jones RN, et al. . Telephone interview for cognitive status: Creating a crosswalk with the Mini-Mental State Examination. Alzheimers Dement. 2009;5(6):492-7. doi:10.1016/j.jalz.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langa KM, Plassman BL, Wallace RB, et al. . The Aging, Demographics, and Memory Study: Study design and methods. Neuroepidemiology. 2005;25(4):181-91. doi:10.1159/000087448 [DOI] [PubMed] [Google Scholar]
- 30.Lindgren N, Rinne JO, Palviainen T, Kaprio J, Vuoksimaa E. Prevalence and correlates of dementia and mild cognitive impairment classified with different versions of the modified Telephone Interview for Cognitive Status (TICS-m). Int J Geriatr Psychiatry. 2019;34(12):1883-91. doi:10.1002/gps.5205 [DOI] [PubMed] [Google Scholar]
- 31.Hoefman RJ, Van Exel NJA, Brouwer WBF. iVICQ: iMTA Valuation of Informal Care Questionnaire. Institute for Medical Technology Assessment. Published May 2013. Accessed May 25, 2022. https://www.imta.nl/assets/uploads/2022/01/iVITO UK version 1.1.pdf [Google Scholar]
- 32.Olazaran J, Aguera-Ortiz L, Argimon JM, et al. . Costs and quality of life in community-dwelling patients with Alzheimer’s disease in Spain: Results from the GERAS II observational study. Int Psychogeriatr. 2017;29(12):2081-93. doi:10.1017/S1041610217001211 [DOI] [PubMed] [Google Scholar]
- 33.Grafova IB, Monheit AC, Kumar R. Income shocks and out-of-pocket health care spending: Implications for single-mother families. J Fam Econ Issues. 2022;43(3):489-500. doi:10.1007/s10834-021-09780-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCammon RJ, Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study. Imputation of cognitive functioning measures: 1992-2016 (Version 1.0). Data description. Survey Research Center University of Michigan. Published April 2022. Accessed May 25, 2022. https://hrsdata.isr.umich.edu/sites/default/files/documentation/data-descriptions/1651088507/COGIMP9218dd.pdf?_ga=2.253004681.1068666510.1685024736-8692.344481613988968 [Google Scholar]
- 35.U.S. Bureau of Labor Statistics. Measuring price change in the CPI: Medical care. Accessed March 9, 2022. https://www.bls.gov/cpi/factsheets/medical-care.htm
- 36.Bucks RS, Ashworth DL, Wilcock GK, Siegfried K. Assessment of activities of daily living in dementia: Development of the Bristol Activities of Daily Living Scale. Age and Ageing. 1996;25(2):113-20. doi:10.1093/ageing/25.2.113 [DOI] [PubMed] [Google Scholar]
- 37.Radloff LS. The CES-D Scale:A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi:10.1177/014662167700100306 [Google Scholar]
- 38.Health and Retirement Study, public use dataset. Produced and distributed by the University of Michigan with funding from the National Institute on Aging; (grant number NIA U01AG009740). Ann Arbor, MI. 2022. Accessed April 7, 2022. [Google Scholar]
- 39.Heeringa SG, Connor JH. Technical description of the Health and Retirement Survey sample design. University of Michigan Institute for Social Research. 1995. Accessed May 25, 2022. https://hrsonline.isr.umich.edu/sitedocs/userg/HRSSAMP.pdf [Google Scholar]
- 40.Zissimopoulos J, Crimmins E, St Clair P. The value of delaying Alzheimer’s disease onset. Forum Health Econ Policy. 2014;18(1):25-39. doi:10.1515/fhep-2014-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware EB, Faul JD, Mitchell CM, Bakulski KM. Considering the APOE locus in Alzheimer’s disease polygenic scores in the Health and Retirement Study: A longitudinal panel study. BMC Med Genomics. 2020;13(1):164. doi:10.1186/s12920-020-00815-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stebbins RC, Noppert GA, Yang YC, Dowd JB, Simanek A, Aiello AE. Association between immune response to cytomegalovirus and cognition in the Health and Retirement Study. Am J Epidemiol. 2021;190(5):786-97. doi:10.1093/aje/kwaa238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beydoun HA, Beydoun MA, Weiss J, et al. . Insomnia as a predictor of diagnosed memory problems: 2006-2016 Health and Retirement Study. Sleep Med. 2021;80: 158-66. doi:10.1016/j.sleep.2021.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley AS, McGarry K, Bollens-Lund E, et al. . Residential setting and the cumulative financial burden of dementia in the 7 years before death. J Am Geriatr Soc. 2020;68(6):1319-24. doi:10.1111/jgs.16414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411-31. doi:10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 46.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607-14. doi:10.1001/archneurol.2011.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo EH, Lee DY, Kim SG, et al. . Validity of the telephone interview for cognitive status (TICS) and modified TICS (TICSm) for mild cognitive imparment (MCI) and dementia screening. Arch Gerontol Geriatr. 2011;52(1):e26-30. doi:10.1016/j.archger.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 48.Cook SE, Marsiske M, McCoy KJ. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22(2):103-9. doi:10.1177/0891988708328214 [DOI] [PMC free article] [PubMed] [Google Scholar]


