Figure 2. Concepts of wild-type (WT) p53 activation and small-molecule-based mutant p53 reactivation.
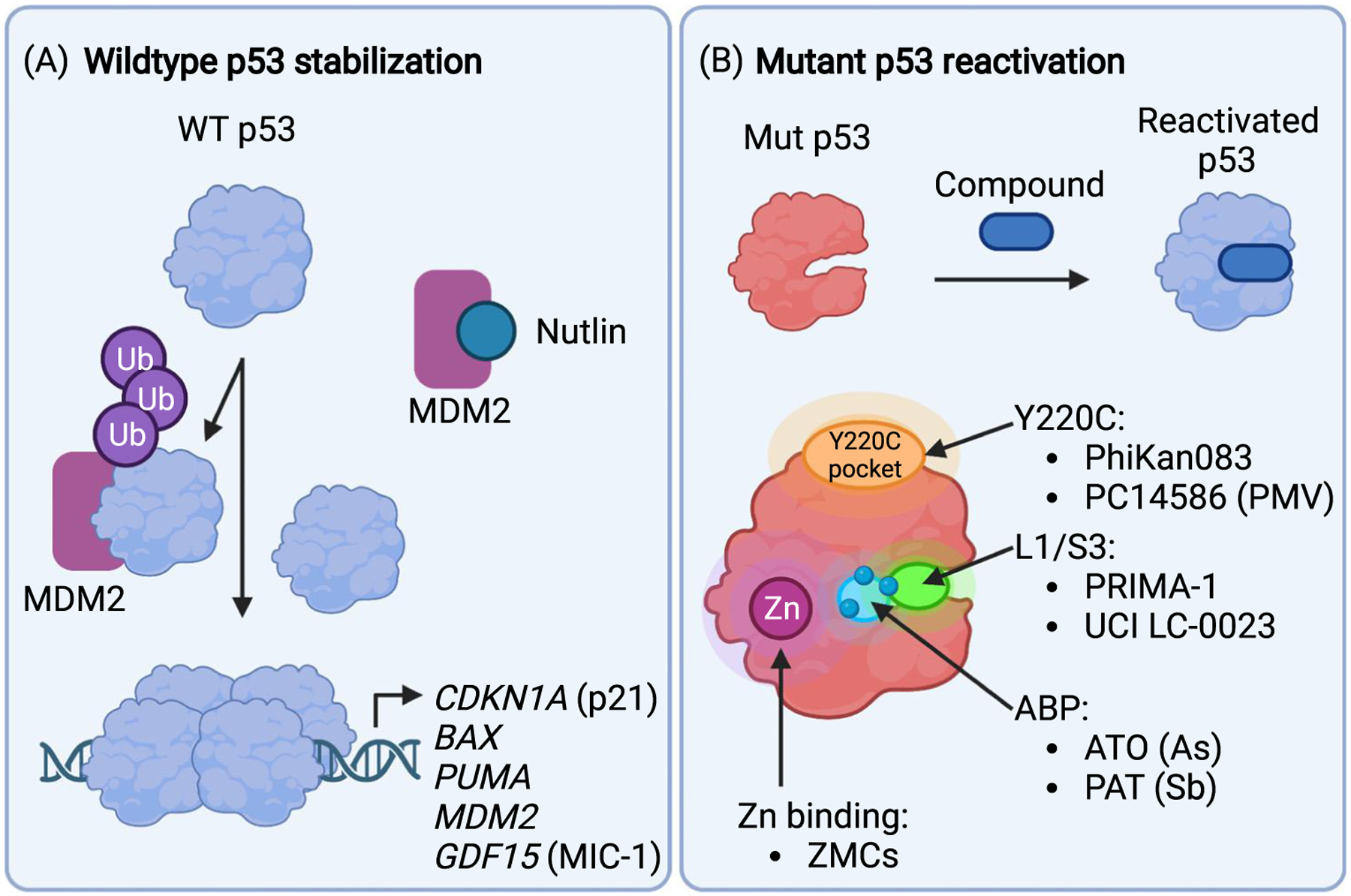
(A) Many tumors with WT p53 overexpress the ubiquitin (Ub) ligase MDM2. This leads to rapid degradation of p53 by the proteasome and inactivation of tumor suppression. Nutlins and other small-molecule MDM2 antagonists block the MDM2/p53 interaction resulting in accumulation of p53, assembly of the active p53 tetramer, and induction of genes that trigger cell-cycle arrest and cell death. (B) Small molecules that bind mutant p53 and stabilize a WT-like conformation are known as corrector or reactivation drugs. Several small molecules with different proposed binding modes and mechanisms have been identified. PhiKan0083 and PC14586 bind to a cryptic crevice created by the Y220C mutation. Zinc metallochaperones (ZMCs) act as zinc ionophores to facilitate zinc binding to p53 mutants with reduced zinc binding capacity. Arsenicals (arsenic trioxide, ATO) and antimonials (potassium antimony tartrate, PAT) release arsenic and antimony, respectively, which are complexed by three specific cysteine residues (three blue circles) that comprise the C124–C135–C141 pocket (light blue region). Arsenic and antimony binding to this pocket (ABP) stabilizes an active conformation of p53 mutants. A degradation product of PRIMA-1 covalently attaches to several cysteines and reactivates p53 mutants, and UCI-LC0023 binds noncovalently to a transiently open pocket (L1/S3 pocket, green area) in the same region to restore activity to p53 mutants. Note that C124 is located in the L1/S3 pocket. Created with BioRender.com.
