Abstract
Ripretinib is a tyrosine kinase inhibitor that was approved by the United States FDA in 2020 for treatment of advanced gastrointestinal stromal tumor (GIST) in patients who received prior treatment with three or more tyrosine kinase inhibitors. In this case report, we show the durable clinical benefit achieved in a patient with GIST by using ripretinib and repeated timely surgical resection of limited disease progression. The total time on ripretinib was 43 months which is longer than the current reported data from ripretinib clinical trials. Such approach for using multi-disciplinary disease management can improve the durability of response to tyrosine kinase inhibitors, including ripretinib, and associated clinical outcomes.
Keywords: ripretinib, precision oncology, sarcoma, surgery
INTRODUCTION
Gastrointestinal Stromal Tumor (GIST) is the most common mesenchymal tumor of the gastrointestinal tract [1]. Most cases of GIST harbor a mutation in either KIT or PDGFRA gene [2–4]. Use of targeted therapies has revolutionized treatment of GIST starting with imatinib in 2002 which has controlled tumor growth in around 80% of patients [5]. Tyrosine kinase inhibitors (TKIs) such as sunitinib and regorafenib are used post-imatinib and have conferred modest clinical benefit [6–8]. However, recurrent GIST post imatinib has remained a challenge even with newer agents and multiple combination therapies.
Ripretinib is a novel TKI that was FDA approved in 2020 for treatment of advanced GIST in patients who received prior treatment with three or more TKIs, including imatinib [9]. Ripretinib is a “switch-control” kinase inhibitor that forces the activation loop (or activation “switch”) into an inactive conformation and inhibits broad spectrum of KIT and PDGFRA mutant variants including activation loop mutations [10]. In addition to an acceptable safety profile, ripretinib has been shown in the INVICTUS randomized phase 3 trial (NCT03353753) to improve the median progression-free survival (PFS) and overall survival (OS) compared to placebo control (6.3 versus 1 month for PFS; and 18.2 vs. 6.3 months for OS) [11, 12]. Herein, we describe the use of ripretinib in a patient with recurrent GIST harboring a KIT exon 11 mutation following treatment with multiple TKIs. The patient was managed with ripretinib and surgical resection of progressing lesions at multiple time points which led to extended clinical benefit.
CASE PRESENTATION
A Hispanic female in her late 20s presented with left lower quadrant pain initially attributed to an ovarian cyst. A workup with computed tomography (CT) evaluation, revealed an incidental 6 cm duodenal mass with central necrosis. She underwent exploratory laparotomy with resection of the mass which was diagnosed as GIST with high malignant potential and positive immunohistochemistry for c-KIT. She was followed with close observation for 3 years before a recurrence was noted in the surgical bed in CT scans. The patient received a pre-operative 5-day course of imatinib as part of a clinical trial followed by surgery and adjuvant imatinib (600 mg daily) for 2 years.
Nearly two years after stopping imatinib, surveillance CT imaging demonstrated local recurrence and left retroperitoneal lymphadenopathy. Patient was restarted on imatinib (400 mg daily) and had disease control for four more years, until restaging scans showed progressing disease in paraaortic lymph node and surgical bed. The imatinib dose was increased to 800 mg daily but ultimately failed to control tumor growth, and the patient experienced disease progression. Treatment was switched to sunitinib (37.5 mg daily), a multi-kinase inhibitor with activity against PDGFR and c-KIT, with subsequent disease control. A year later, her tumor showed mixed response while on sunitinib and a multi-disciplinary tumor board recommended surgical resection of progressing lesions. Patient underwent surgical resection and after surgery she continued sunitinib for two years until she developed disease progression in abdominal lymph nodes.
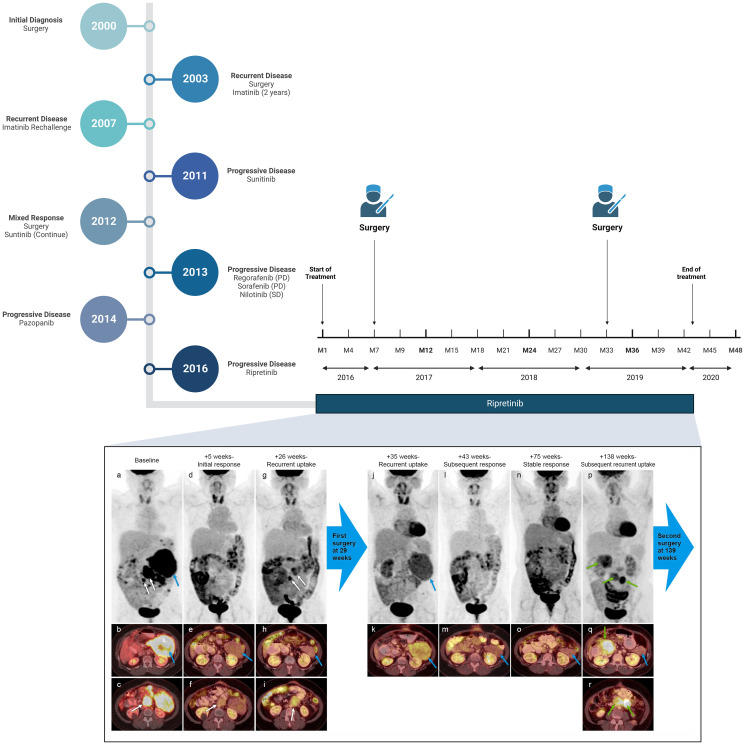
The patient was started on regorafenib (120 mg), a multi-kinase inhibitor of VEGFR1-3, PDGFRB, FGFR1, RAF and KIT, and subsequently sorafenib (800 mg daily), a multi-kinase inhibitor of VEGF, PDGFRB, and KIT, but neither drugs was able to control her disease (best response was progressive disease). She was initiated on nilotinib (800 mg daily) and continued treatment for one year when her scans started to show evidence of disease progression. Pazopanib was administered for two years with partial response but unfortunately her disease progressed (Figure 1).
Figure 1. Clinical history of the patient and PET/CT images of patient while on Ripretinib therapy.
Representative serial PET/CT imaging studies from baseline (prior to initiation of ripretinib) to immediately before her second surgery are illustrated in the figure. The MIP images from PET are illustrated in the top row and fused PET/CT images are illustrated in subsequent rows. The baseline PET/CT (a–c) shows multiple FDG-avid peritoneal/retroperitoneal implants of which 3 target areas are annotated (dominant lesion in the left upper quadrant is annotated with blue arrow and two smaller lesions are annotated with white arrows). Follow-up PET/CT at 5 weeks (d–f) shows decreased size and uptake of all implants including the target lesions. Follow up PET/CT at 26 weeks (g–i) shows recurrent uptake in two smaller target lesions near the midline (white arrows) and persistent response in the dominant lesion in the left upper quadrant (blue arrow). Only one of the two smaller lesions is illustrated with axial fused PET/CT images c, f and i. These two smaller lesions showing recurrent uptake were resected at ~29 weeks. Follow-up PET/CT images after initial surgery and 35 weeks from baseline (j, k) showed recurrent uptake in the previously responding dominant left upper quadrant implant (blue arrows), which resolved on the subsequent follow-up PET/CTs at 43 weeks (l, m) and 75 weeks (n, o). The disease then slowly progressed over the next few studies with eventual development of 3 new areas of uptake illustrated on 138 week follow-up PET/CT (p–r). The new sites of uptake are annotated with green arrows. Note that the dominant left upper quadrant implant shows persistent response at the 138 week follow-up study (blue arrow). The patient then underwent second surgical resection and subsequent follow-up imaging (not illustrated here) showed residual disease that further responded to treatment and then progressed with development of new liver metastases.
At this time, the patient presented to the phase I program to explore the options of enrollment in a clinical trial. Based on the tumor KIT alteration, she was enrolled on investigational treatment with ripretinib (DCC-2618; NCT02571036). Baseline PET/CT showed involvement of multiple peritoneal implants and abdominal lymph nodes. Following treatment with ripretinib, initial re-staging with PET/CT scans showed partial response (−34% change in target lesions per RECIST) that was associated with clinical benefit in the form of resolving abdominal pain and subjective feeling of clinical improvement. Four months later, scans showed progressive disease in two smaller lesions with stable disease in the dominant target lesions. Given the clinical benefit and subjective improvement of pain, non-responding lesions were surgically resected. After surgery, patient was re-initiated on ripretinib and achieved additional shrinkage resulting in partial response (−32% change in target lesions). Response to ripretinib was maintained for 26 months until she developed progression in target lesions. Over the following few months, dose escalation from 100 mg BID to 150 mg BID was performed, as specified on the trial to control increase in the size of target lesions. This Intrapatient dose escalation resulted in clinical benefit in the form of improved pain and quality of life. However, eventually, the patient developed anemia and gastrointestinal bleeding that was attributed to development of a large fungating duodenal mass. She underwent surgical resection of the mass and then she continued treatment with ripretinib 150 mg BID given the lack of further systemic treatment options. Tissue next-generation sequencing (NGS) by that time confirmed the presence of KIT exon 11 p.N567_Q575delinsKEV (compared to retrospective analysis of older tissue blocks that showed both KIT exon 13 N665T and KIT exon 11 p.N567_Q575delinsKEV). Patient continued treatment for another 10 months when her CT scans, unfortunately, showed disease progression. Liquid biopsy NGS at time of progression showed the same mutational profile of KIT exon 11 p.N567_Q575delinsKEV. Patient was taken off ripretinib and enrolled onto a clinical trial with another investigational agent (Figure 1).
DISCUSSION
Recurrent/relapsed GIST is a clinically challenging disease. Herein, we present a report of successful disease control of GIST (associated with KIT exon 11 mutation) utilizing treatment with the novel switch-kinase inhibitor ripretinib alternating with surgery to control resistant/non-responding lesions. Current data on ripretinib come from the INVICTUS randomized controlled trial which suggested a median PFS of 6.3 months (95% CI 4.6–6.9) for patients receiving active treatment. In the phase 1 trial in which patient was enrolled, the study yielded a confirmed objective response rate (ORR) of 11.3% (n = 16/142). This rate varied from 7.2% (n = 6/83; fourth line or greater) to 19.4% (n = 6/31; second line) depending on the treatment line. Additionally, the median PFS ranged from 5.5 months (fourth line or greater) to 10.7 months (second line), as assessed by the study investigators [13]. In our patient, the total time on therapy with ripretinib was 43 months with the use of timely surgery for limited progression. Similar strategy of using surgery for limited progression or residual disease is often used with imatinib and is supportive by multiple retrospective reports.
Following progression on multiple TKIs (including imatinib, sunitinib, regorafenib, sorafenib, nilotinib, and pazopanib), our patient started treatment with ripretinib, and continued treatment as restaging scans showed partial response or stable disease with clinical benefit. Ripretinib has an inhibitory effect on KIT tyrosine kinase which was part of this patient’s tumor mutational profile (KIT exon 13 N655T and KIT exon 11 p.N567_Q575delinsKEV). It was approved by the FDA in 2020 after data from the INVICTUS international multicenter placebo-controlled phase 3 trial which showed a greater objective response rate as well as markedly improved median PFS compared to placebo [11].
Dose escalation has been used at some point in our patient, as a tool to overcome resistance, and led to control of progressing disease. Adaptive and evasive resistance with TKIs has been previously reported with multiple drugs [14–17]. Such resistance due to decreased sensitivity to TKIs can be overcome through intrapatient dose escalation. For example, in patients treated with osimertinib for EGFR mutated non-small cell lung cancer (NSCLC), intracranial and leptomeningeal progression was controlled by increasing the dose from 80 mg to 160 mg QD in anecdotal studies [18–20]. In fact, dose escalation to overcome disease progression has been reported in GIST with other TKIs [21] and also with ripretinib [22, 23].
Another mechanism of resistance for TKIs is the development of novel mutations. For example, additional mutations in KIT and PDGFRA were detected post-treatment in patients with GIST progressing on TKI therapy [24]. The clonal evolution of cancer can be challenging particularly with multi-kinase inhibitors since it can lead to isolated subclonal progression in heterogenous tumor sites [25–28]. Such localized progression, as seen in our patient, can be managed through surgical resection of non-responding lesions [29, 30]. Surgical management of isolated progression was performed twice during the course of treatment with ripretinib in our patient and led to a prolonged clinical benefit.
Investigational therapy with targeted therapeutic agents should allow intra-patient dose escalation, treatment beyond progression for clinical benefit, and local control of non-target lesions especially if the patient is deriving clinical benefit. Multi-disciplinary care with surgery and targeted therapy can extend duration on treatment with ripretinib.
ACKNOWLEDGMENTS AND FUNDING
Vivek Subbiah (VS) reports employment with UT MD Anderson Cancer Center at the time of submission and reports employment by Sarah Cannon Research Institute at the time of acceptance. VS is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center. VS acknowledges the support of The Jacquelyn A. Brady Fund. VS is supported by a US National Institutes of Health (NIH) grant (No. R01CA242845 and R01CA273168); MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas (No. RP1100584), the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (No. 1U01 CA180964), NCATS (Center for Clinical and Translational Sciences) Grant (No. UL1 TR000371), and the MD Anderson Cancer Center Support Grant (No. P30 CA016672). The included figure was created with https://www.biorender.com/.
Author contributions
Authors have contributed to the conception and writing of this manuscript. All authors have approved the final manuscript draft.
CONFLICTS OF INTEREST
MG and SY have no conflicts of interest. FJ is an employee and has leadership position in Monte Rosa Therapeutics; and has ownership interest in Monte Rosa Therapeutics (stocks/options) and Cardiff Oncology (stocks). NS is on the advisory board of Deciphera, Bayer, and Aadi Biosciences. KKH is on the medical advisory board of ArmadaHealth and Astrazeneca; and has received research funding (to institution) from Cairn Surgical, Eli Lilly & Co., and Lumicell. VS at the time of submission reports research funding from Novartis to conduct clinical trials; and other grant support for clinical trials from AbbVie, Agensys, Inc., Alfasigma, Altum, Amgen, Bayer, BERG Health, Blueprint Medicines Corporation, Boston Biomedical, Inc., Boston Pharmaceuticals, Celgene Corporation, D3 Bio, Inc., Dragonfly Therapeutics, Inc., Exelixis, Fujifilm, GlaxoSmithKline, Idera Pharmaceuticals, Inc., Incyte Corporation, Inhibrx, Loxo Oncology/Eli Lilly, MedImmune, MultiVir, Inc., NanoCarrier, Co., National Comprehensive Cancer Network, NCI-CTEP, Novartis, PharmaMar, Pfizer, Relay, Therapeutics, Roche/Genentech, Takeda, Turning Point Therapeutics, UT MD Anderson Cancer Center, and Vegenics Pty. Ltd.; as well as travel support from ASCO, ESMO, Helsinn Healthcare, Incyte Corporation, Novartis, and PharmaMar; and reports consultancy or advisory board participation for Helsinn Healthcare, Incyte Corporation, Loxo Oncology/Eli Lilly, MedImmune, Novartis, QED Therapeutics, Relay Therapeutics, Daiichi-Sankyo, and R-PharmUS; and other relationship with Medscape.
Ethical statement and consent
Authors obtained informed consent from the patient for sharing information related to the case including clinical and imaging data which have been de-identified during manuscript writing.
REFERENCES
- 1.Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016; 40:39–46. 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998; 279:577–80. 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, McGreevey L, Haley A, Town A, Heinrich MC. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol. 2002; 160:1567–72. 10.1016/S0002-9440(10)61103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003; 299:708–10. 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, Kiese B, Eisenberg B, Roberts PJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003; 21:4342–49. 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 6.Kelly CM, Gutierrez Sainz L, Chi P. The management of metastatic GIST: current standard and investigational therapeutics. J Hematol Oncol. 2021; 14:2. 10.1186/s13045-020-01026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, et al. Gastrointestinal stromal tumours: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018; 29:iv267. 10.1093/annonc/mdy320. [DOI] [PubMed] [Google Scholar]
- 8.Casali PG, Zalcberg J, Le Cesne A, Reichardt P, Blay JY, Lindner LH, Judson IR, Schöffski P, Leyvraz S, Italiano A, Grünwald V, Pousa AL, Kotasek D, et al. Ten-Year Progression-Free and Overall Survival in Patients With Unresectable or Metastatic GI Stromal Tumors: Long-Term Analysis of the European Organisation for Research and Treatment of Cancer, Italian Sarcoma Group, and Australasian Gastrointestinal Trials Group Intergroup Phase III Randomized Trial on Imatinib at Two Dose Levels. J Clin Oncol. 2017; 35:1713–20. 10.1200/JCO.2016.71.0228. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon S. Ripretinib: First Approval. Drugs. 2020; 80:1133–38. 10.1007/s40265-020-01348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BD, Kaufman MD, Lu WP, Gupta A, Leary CB, Wise SC, Rutkoski TJ, Ahn YM, Al-Ani G, Bulfer SL, Caldwell TM, Chun L, Ensinger CL, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell. 2019; 35:738–51.e9. 10.1016/j.ccell.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Blay JY, Serrano C, Heinrich MC, Zalcberg J, Bauer S, Gelderblom H, Schöffski P, Jones RL, Attia S, D’Amato G, Chi P, Reichardt P, Meade J, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020; 21:923–34. 10.1016/S1470-2045(20)30168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Mehren M, Heinrich MC, George S, Zalcberg JR, Bauer S, Gelderblom H, Schoffski P, Serrano C, Jones RL, Attia S, D’Amato G, Chi P, Reichardt P, et al. Ripretinib as ≥4th-line treatment in patients with advanced gastrointestinal stromal tumor: Long-term update from the phase III INVICTUS study. Ann Oncol. 2021; 32:S1120–21. 10.1016/j.annonc.2021.08.870. [DOI] [Google Scholar]
- 13.Janku F, Abdul Razak AR, Chi P, Heinrich MC, von Mehren M, Jones RL, Ganjoo K, Trent J, Gelderblom H, Somaiah N, Hu S, Rosen O, Su Y, et al. Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J Clin Oncol. 2020; 38:3294–303. 10.1200/JCO.20.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, Rodrigues MJ, Rousseau B, Blanchet B, Goldwasser F. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012; 30:2046–49. 10.1007/s10637-011-9764-8. [DOI] [PubMed] [Google Scholar]
- 15.Hughes T, Hochhaus A. Clinical strategies to achieve an early and successful response to tyrosine kinase inhibitor therapy. Semin Hematol. 2009; 46:S11–15. 10.1053/j.seminhematol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Kuczynski EA, Lee CR, Man S, Chen E, Kerbel RS. Effects of Sorafenib Dose on Acquired Reversible Resistance and Toxicity in Hepatocellular Carcinoma. Cancer Res. 2015; 75:2510–19. 10.1158/0008-5472.CAN-14-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotink KJ, Broxterman HJ, Labots M, de Haas RR, Dekker H, Honeywell RJ, Rudek MA, Beerepoot LV, Musters RJ, Jansen G, Griffioen AW, Assaraf YG, Pili R, et al. Lysosomal sequestration of sunitinib: a novel mechanism of drug resistance. Clin Cancer Res. 2011; 17:7337–46. 10.1158/1078-0432.CCR-11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein IM, Roisman LC, Keren-Rosenberg S, Dudnik J, Nechushtan H, Shelef I, Fuchs V, Kian W, Peled N. Dose escalation of osimertinib for intracranial progression in EGFR mutated non-small-cell lung cancer with brain metastases. Neurooncol Adv. 2020; 2:vdaa125. 10.1093/noajnl/vdaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cordova C, Chi AS, Chachoua A, Kondziolka D, Silverman JS, Shepherd TM, Jain R, Snuderl M. Osimertinib Dose Escalation Induces Regression of Progressive EGFR T790M-Mutant Leptomeningeal Lung Adenocarcinoma. J Thorac Oncol. 2017; 12:e188–90. 10.1016/j.jtho.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Tsang MW. Osimertinib 160 mg daily for advanced non-small cell lung cancer with leptomeningeal metastasis: A case report. Asia Pac J Clin Oncol. 2019. (Suppl 6); 15:5–7. 10.1111/ajco.13246. [DOI] [PubMed] [Google Scholar]
- 21.Zalcberg JR, Desai J. Dose optimization of tyrosine kinase inhibitors to improve outcomes in GIST. Asia Pac J Clin Oncol. 2012; 8:43–52. 10.1111/j.1743-7563.2011.01491.x. [DOI] [PubMed] [Google Scholar]
- 22.Zalcberg JR, Heinrich MC, George S, Bauer S, Schöffski P, Serrano C, Gelderblom H, Jones RL, Attia S, D’Amato G, Chi P, Reichardt P, Somaiah N, et al. Clinical Benefit of Ripretinib Dose Escalation After Disease Progression in Advanced Gastrointestinal Stromal Tumor: An Analysis of the INVICTUS Study. Oncologist. 2021; 26:e2053–60. 10.1002/onco.13917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George S, Chi P, Heinrich MC, von Mehren M, Jones RL, Ganjoo K, Trent J, Gelderblom H, Razak AA, Gordon MS, Somaiah N, Jennings J, Meade J, et al. Ripretinib intrapatient dose escalation after disease progression provides clinically meaningful outcomes in advanced gastrointestinal stromal tumour. Eur J Cancer. 2021; 155:236–44. 10.1016/j.ejca.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang G, Bae BN, Sohn BS, Pyo JS, Kang GH, Kim KM. Detection of KIT and PDGFRA mutations in the plasma of patients with gastrointestinal stromal tumor. Target Oncol. 2015; 10:597–601. 10.1007/s11523-015-0361-1. [DOI] [PubMed] [Google Scholar]
- 25.McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell. 2017; 168:613–28. 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Losic B, Craig AJ, Villacorta-Martin C, Martins-Filho SN, Akers N, Chen X, Ahsen ME, von Felden J, Labgaa I, DʹAvola D, Allette K, Lira SA, Furtado GC, et al. Intratumoral heterogeneity and clonal evolution in liver cancer. Nat Commun. 2020; 11:291. 10.1038/s41467-019-14050-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res. 2013; 19:5310–19. 10.1158/1078-0432.CCR-13-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unk M, Jezeršek Novaković B, Novaković S. Molecular Mechanisms of Gastrointestinal Stromal Tumors and Their Impact on Systemic Therapy Decision. Cancers (Basel). 2023; 15:1498. 10.3390/cancers15051498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S. Managing progressive disease in patients with GIST: factors to consider besides acquired secondary tyrosine kinase inhibitor resistance. Cancer Treat Rev. 2012; 38:467–72. 10.1016/j.ctrv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Raut CP, Posner M, Desai J, Morgan JA, George S, Zahrieh D, Fletcher CD, Demetri GD, Bertagnolli MM. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol. 2006; 24:2325–31. 10.1200/JCO.2005.05.3439. [DOI] [PubMed] [Google Scholar]