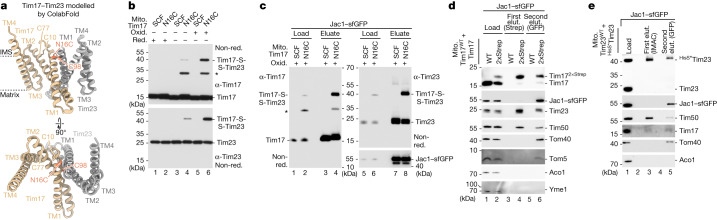
Fig. 2. Lateral transmembrane cavities face toward opposite sides of the Tim17–Tim23 dimer.
a, ColabFold structural protein complex model of Tim17 (tan) and Tim23 (grey) heterodimers. Tan, endogenous intramolecular disulfide bond formed between Tim17 Cys10 (C10) and Cys77 (C77); orange, Tim17 Cys16 (N16C) and Tim23 Cys98 (C98) forming an intermolecular disulfide bond. b, In organello disulfide bond formation between Tim17 and Tim23. Mitochondria isolated from yeast strains expressing SCF Tim17 or Tim17 N16C were left untreated or treated with the reductant dithiothreitol (DTT; red) or oxidant 4,4′-dipyridyl disulfide (4-DPS; Oxid.) followed by SDS–PAGE. Tim17-S-S-Tim23, disulfide-linked Tim17 and Tim23. *Unidentified Tim17-specific conjugate. c, Import of Jac1–sfGFP into SCF Tim17 or N16C mitochondria followed by oxidation with 4-DPS. Jac1–sfGFP-containing TOM–TIM23 supercomplexes were isolated with a GFP nanobody. Load (1%) and eluate (100%) fractions were analysed by SDS–PAGE and immunodecoration. d,e, Import of Jac1–sfGFP into wild-type or Tim172xStrep (d) and HisSumo*Tim23 mitochondria (e). TIM23 complexes were isolated by tandem purification first using streptavidin (Strep; d) or immobilized metal affinity chromatography (IMAC; e) followed by purification of Jac1–sfGFP-containing TOM–TIM23 supercomplexes using a GFP nanobody. Fractions were analysed by SDS–PAGE and immunodecoration. Load, 0.25%; first elut., first elution (2.5%); second elut., second elution (100%).