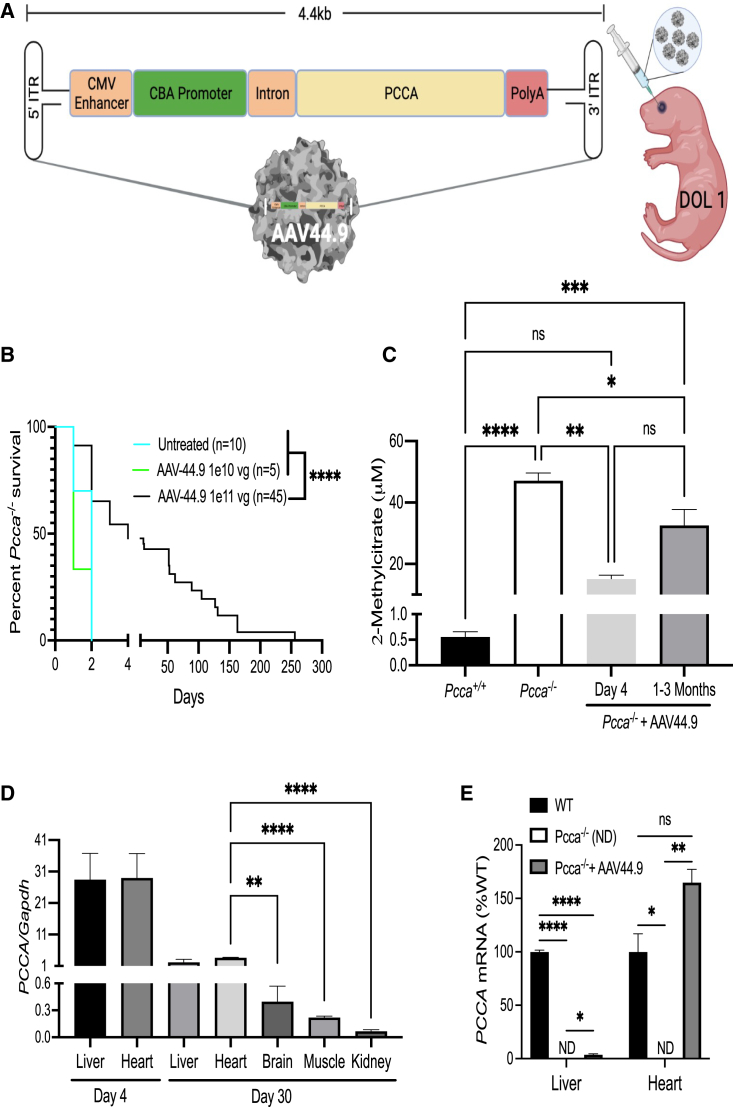
Figure 4.
AAV44.9 gene delivery of PCCA rescues Pcca−/− mice from neonatal lethality
(A) Schematic of the recombinant AAV vector genome (pAAV-CBA-PCCA) packaged with the AAV44.9 capsid (CMV, cytomegalovirus; CBA, chicken β-actin). Pcca−/− pups received a retro-orbital injection of 1e10 vg or 1e11 vg of AAV44.9-CBA-PCCA on day of life 1 (DOL 1). (B) Survival curve comparing the viability of Pcca−/− mice treated with AAV44.9 at doses of 1e10 vg or 1e11 vg with untreated Pcca−/− mice. (C) Plasma 2-methylcitrate in untreated neonatal Pcca−/− mice and Pcca−/− mice 4 days and 1–3 months after treatment with AAV44.9 at 1e11 vg. (D) Vector biodistribution in liver, heart, brain skeletal muscle, and kidneys from Pcca−/− treated mice (n = 3) 4 days and 1 month post treatment, as determined by ddPCR. (E) PCCA and Pcca mRNA expression in the liver and heart (WT, n = 3, 3; PA, n = 2, 1; AAV44.9, n = 3, 3), shown as a percentage of WT Pcca mRNA expression normalized to Actb (liver) or Gapdh (heart). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; ns, not significant; ND, not detected.