Abstract
The 2-U penicillin and 1-μg oxacillin discs proposed for screening meningococci for susceptibility to penicillin were evaluated by using MICs measured by the E test. The discs yielded unacceptably high frequencies of misclassification of susceptibility category and should be abandoned in favor of MIC estimations. An agreed breakpoint for reduced penicillin susceptibility in meningococci is needed for the E test.
Penicillin susceptibility testing of meningococci has long been a difficult area, and authorities recommend MIC determination as the method of first choice (10). Nevertheless, the traditional disc diffusion test has remained in use as a practical and relatively inexpensive method. Campos and coworkers have argued that problems of interpretation encountered with regular 10-U penicillin discs may be reduced by using 2-U penicillin or 1-μg oxacillin discs (2, 3). These discs were introduced in Israel in 1992 and 1995, respectively, and since introduction of regular MIC measurements obtained by using the E test in 1995, sufficient strains have been examined to permit an adequate analysis of their value.
The E test has been shown in several studies to be an acceptably accurate method for determining MICs for Neisseria meningitidis (1, 4–6). Despite the E test’s high cost, the relatively small number of cases of meningococcal disease encountered in Israel each year makes it an attractively practical option for MIC estimations.
The present study was conducted from January 1995 through July 1997 with 133 consecutive clinical isolates of N. meningitidis submitted to the Israel National Center for Meningococci at Tel Hashomer. Laboratories isolating N. meningitidis from patients are required by law to submit the isolates to the Center for characterization. Internal audit has revealed that 95% of these N. meningitidis isolates are received at the Center, where they are serogrouped and serotyped, their antibiotic susceptibilities are tested, and they are stored at −70°C.
Disc diffusion testing was performed by using 2-U penicillin and 1-μg oxacillin discs (Sanofi Diagnostics Pasteur, Marnes La Coquette, France) according to National Committee for Clinical Laboratory Standards recommendations (7). Blood (5%) was added to the Mueller-Hinton agar. Data on record at the Israel National Center for Meningococci from previous years, obtained with 10-U penicillin discs, showed that Mueller-Hinton agar without blood (MH) gave significantly larger zone diameters than Mueller-Hinton agar with 5% blood (MHB). A total of 278 strains examined with MH gave an average inhibition zone of 42.4 mm (median, 40 mm; standard deviation, 7.4), while 315 strains tested on MHB gave a mean of 35.6 mm (median, 35 mm; standard deviation, 4.7) (P < 0.0001 by the t test). Others have also found unsupplemented Mueller-Hinton media wanting, so various additions have been used, including blood (4, 8, 9). The definitions of reduced susceptibility used for the analyses were those proposed by Campos et al. (3): inhibition zone diameters of ≤26 mm around the 2-U penicillin disc and ≤10 mm around the 1-μg oxacillin disc.
The penicillin E test (AB Biodisk, Solna, Sweden) was performed according to the manufacturer’s instructions, on MHB. With few exceptions, tests were performed by the same individual. The conventional penicillin MIC of ≥0.1 μg/ml was used to denote strains with reduced susceptibility. This has limitations in respect to the E test, which are elaborated below.
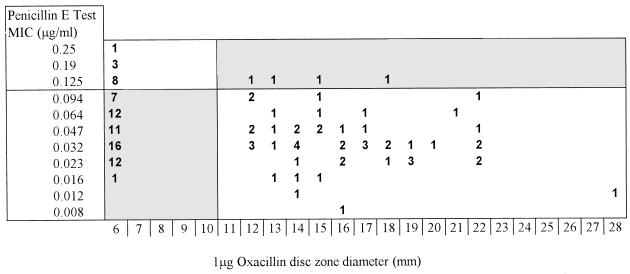
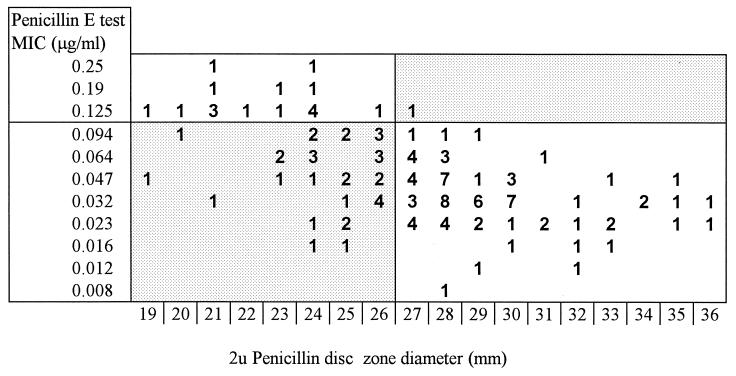
Figures 1 and 2 indicate the relationships between disc diffusion zone diameters and MICs. The data show clearly that the 1-μg oxacillin disc was a poor predictor of penicillin susceptibility in this in-use evaluation. Four of 16 strains (25.0%) for which penicillin MICs were ≥0.125 μg/ml were misclassified as susceptible by the disc, whereas 59 of 112 sensitive strains (52.7%) were misclassified as having reduced susceptibility. The 2-U penicillin disc showed less “false susceptibility”: 1 of 18 (5.6%) strains with reduced susceptibility were misclassified as susceptible, but 34 of 115 (29.6%) susceptible isolates were judged resistant.
FIG. 1.
Zone diameter measurements obtained by using 1-μg oxacillin discs versus MICs of 128 strains of N. meningitidis.
FIG. 2.
Zone diameter measurements obtained by using 2-U penicillin discs versus MICs of 133 strains of N. meningitidis.
These data were based on the conventional breakpoint, as mentioned above. The scale of MICs provided by the E test includes a value of 0.094 μg/ml. It would appear reasonable to take MICs at this value as representing organisms with reduced penicillin susceptibility, since it is very close to the 0.1-μg/ml cutoff. Citing pharmacokinetic considerations, Hughes et al. (4) defined reduced penicillin susceptibility as a MIC of >0.06 to 1 μg/ml. With 0.094 μg/ml as the breakpoint, the oxacillin disc method would have resulted in the misclassification of 8 of 27 of the less susceptible strains as susceptible (29.6%). Similarly, the rate of misclassification would also have been appreciably higher for the 2-U penicillin disc (4 of 29 isolates [13.8%]).
Other investigators have also found the oxacillin disc to be grossly unreliable, with the 2-U penicillin disc being more useful (9), although for the organisms examined, different zone diameter cutoff points were suggested. Our data showed clearly that, in our hands, recommended methods for disc diffusion determination of penicillin susceptibility resulted in unacceptably high frequencies of misclassification of the susceptibility category and should therefore be abandoned in favor of MIC estimations. For some laboratories, the E test will be a practical method. As shown in our data, an appreciable number of strains straddle the 0.094- to 0.125-μg/ml MIC range, suggesting that the question of an agreed E-test breakpoint for reduced penicillin susceptibility also needs to be further addressed if data from different centers are to be compared.
(This study was presented in part at the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada, 28 September to 1 October 1997.)
REFERENCES
- 1.Abadi F J, Yakubu D E, Pennington T H. Antimicrobial susceptibility of penicillin-sensitive and penicillin-resistant meningococci. J Antimicrob Chemother. 1995;35:687–690. doi: 10.1093/jac/35.5.687. [DOI] [PubMed] [Google Scholar]
- 2.Campos J, Mendelman P M, Sako M U, Chaffin D O, Smith A L, Saez-Nieto J A. Detection of relatively penicillin G-resistant Neisseria meningitidis by disk susceptibility testing. Antimicrob Agents Chemother. 1987;31:1478–1482. doi: 10.1128/aac.31.10.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos J, Trujillo G, Seuba T, Rodriguez A. Discriminative criteria for Neisseria meningitidis isolates that are moderately susceptible to penicillin and ampicillin. Antimicrob Agents Chemother. 1992;36:1028–1031. doi: 10.1128/aac.36.5.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes J H, Biedenbach D J, Erwin M E, Jones R N. E test as susceptibility test and epidemiologic tool for evaluation of Neisseria meningitidis isolates. J Clin Microbiol. 1993;31:3255–3259. doi: 10.1128/jcm.31.12.3255-3259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koeck J L, Cavallo J D, Fabre R, Crenn Y, Chapalain J C, Meyran M. Value of the E-test for the determination of the susceptibility to antibiotics of Neisseria meningitidis. Pathol Biol. 1994;42:465–467. [PubMed] [Google Scholar]
- 6.Marshall S A, Rhomberg P R, Jones R N. Comparative evaluation of etest for susceptibility testing Neisseria meningitidis with eight antimicrobial agents. An investigation using U.S. Food and Drug Administration regulatory criteria. Diagn Microbiol Infect Dis. 1997;27:93–97. doi: 10.1016/s0732-8893(96)00223-4. [DOI] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A5. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 8.Pascual A, Joyanes P, Martinez-Martinez L, Suarez A I, Perea E J. Comparison of broth microdilution and E-test for susceptibility testing of Neisseria meningitidis. J Clin Microbiol. 1996;34:588–591. doi: 10.1128/jcm.34.3.588-591.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Trallero E, Gomez N, Garcia-Arenzana J M. E test as susceptibility test for evaluation of Neisseria meningitidis isolates. J Clin Microbiol. 1994;32:2341–2342. doi: 10.1128/jcm.32.9.2341-2342.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tenover F C. Antimicrobial susceptibility testing of Neisseria meningitidis. Clin Microbiol Newsl. 1993;15:37–38. [Google Scholar]