Abstract
Background
This systematic review aimed to conduct a complete investigation of the demographic aspects, clinicopathological features, degrees of epithelial dysplasia, and malignant transformation rate of actinic cheilitis.
Methods
The study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered in the International Prospective Register of Systematic Reviews (CRD42020201254). A search without year and language restrictions was performed using PubMed/MEDLINE, Embase, Virtual Health Library, Scopus, Web of Science, and gray literature. Studies that provided information on patients with actinic cheilitis were included, excluding those with general information on other diseases or other types of cheilitis. Risk of bias was explored using the Joanna Briggs Institute tool. Narrative and quantitative data syntheses were performed using meta-analyses and subgroup analyses. Association tests were also performed.
Results
Thirteen studies (728 patients) were included. The most prevalent clinical signs were dryness (99%), blurred demarcation between the lip vermilion and skin (82%), scaling (69%), and atrophy (69%). Regarding epithelial dysplasia, a prevalence of mild dysplasia (34.2%), followed by moderate (27.5%), and severe (14.9%). The malignant transformation rate was 14%. Crusts, ulcerations, and erythematous areas were associated with lip carcinoma (p < 0.001), and scaling was associated with actinic cheilitis (p < 0.001).
Conclusions
This study revealed several features of actinic cheilitis, providing an overview of the disease. It is suggested that new studies help develop policy guides for the standardization of clinical criteria, enabling more rigorous and homogeneous analysis of actinic cheilitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-023-01543-z.
Keywords: Actinic cheilitis, Lip diseases, Lip neoplasms, Early diagnosis
Introduction
Actinic cheilitis (AC) is a potentially malignant disorder that affects the lips and is caused by excessive exposure to ultraviolet (UV) radiation [1]. Clinically, there is a blurred demarcation between the lip vermilion and skin, as well as erythematous areas, scaling, and dryness, especially on the lower lip. Plaques, ulcerations, and crusts may be present in advanced cases. If no action is taken, the lesion can develop into squamous cell carcinoma (SCC) [2–7].
The lesion has a male predilection and affects mainly light-skinned individuals who perform occupational activities outdoors [4, 5], such as farmers, fishermen, bricklayers, and athletes [3]. Moreover, it is associated with the male sex owing to the lower frequency of lip balm use than women, as several of them use lipstick with sunscreen in their daily lives, and this is a protective factor for the lips [2–8].
The lesion tends to be erroneously related to signs of aging owing to its slow and typically asymptomatic evolution, which leads to probable progression to SCC if left untreated [2].
The rate of malignancy varies from 10 to 30% [3, 9, 10]. This is because of the ability of UV radiation to induce changes in proteins and DNA, which can cause epithelial dysplasia [11–13]. The lesion becomes even more evident when associated with smoking and alcohol consumption, in addition to the importance of considering socioeconomic status, lifestyle, and heredity [2].
Although AC has broad clinical features that are not frequently clearly linked to microscopic features, there are currently no widely accepted criteria for this disease. Its diagnosis is neglected since several professionals do not recognize its presence in its early stages and is often confused with signs of aging. In addition, lesions are often masked by cosmetic procedures, such as lip fillers. Alternatively, when noticed, it is usually treated as a simple wound, and ointments are prescribed that, in most cases, do not regress the lesion. Therefore, investigations must be performed to assess the numerous clinical and microscopic features of this lesion and score them in detail.
Thus, this systematic review aimed to conduct a complete investigation of the demographic aspects, clinicopathological features, degrees of epithelial dysplasia, and malignant transformation rate of AC, focusing on its clinical and microscopic aspects. The following question motivated this study: “In patients with AC, what is the prevalence of different clinical and microscopic features?”.
Materials and Methods
Research Question and Eligibility Criteria
A modified PECO strategy was used to formulate the question of this systematic review: (P) patients diagnosed with AC, (E) not applicable, (C) not applicable, and (O) prevalence of various clinical and microscopic features.
Studies that provided information on patients diagnosed with AC were included, excluding studies that provided general information on all potentially malignant oral disorders as well as studies on other types of cheilitis, such as angular, contact, drug-related, glandular, granulomatous, and plasma cell cheilitis. Patients with cheilitis associated with dermatoses and systemic diseases (lupus, lichen planus, pemphigus/pemphigoid, angioedema, and xerostomia) were also excluded.
Regarding the outcome, studies that presented the clinical and microscopic (and/or degree of dysplasia) variables of the lesion, nominally or in scales/scores, were included. Studies that did not report the features punctually and descriptively were not included, considering that the aim of this investigation was to score the features in detail. As a secondary outcome, this study also aimed to identify the rate of malignant transformation of lesions.
Finally, it should be noted that observational, cross-sectional, retrospective cohort, prospective cohort, and case–control studies were included, and case reports, case series, and reviews were excluded.
Protocol and Registration
This systematic review was conducted in accordance with the latest guidelines proposed by the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] and registered in the International Prospective Register of Systematic Reviews (PROSPERO) (protocol registration no.: PROSPERO CRD42020201254). The PRISMA 2020 checklist is present in Supplementary Table S1.
Information Sources and Research Strategy
The following electronic databases were used: PubMed/MEDLINE, Embase (via Elsevier), Virtual Health Library, Scopus, and Web of Science. In addition, gray literature was used based on a manual search of the reference lists of relevant studies and the use of the Brazilian Digital Library of Theses and Dissertations, Google Scholar, and ProQuest. The search strategy included terms related to the population (patients with AC) and outcome (clinical and microscopic features), which were combined with Boolean operators, adapted for use in each of the bibliographic databases, in combination with specific filters. The database search strategy is summarized in Supplementary Table S2. No restrictions on the year of publication or language were employed. All bases were checked for data updates on September 19, 2022.
Selection of Studies
After searching all databases, the retrieved records were transferred to the EndNote Web® reference manager (Clarivate, London, UK) to identify duplicates. Two independent reviewers (M.C.C. and M.G.Q.H.) read the titles and abstracts of each article and excluded studies that did not meet the eligibility criteria. With these pre-selected studies, reading of the full texts was performed to analyze which studies precisely met the established criteria. Any discrepancies were resolved through discussion with a third reviewer (C.M.F.R.). The inter-examiner κ value [15] was > 0.80 in both stages.
Data Collection and Extracted Data
Study data were independently extracted by two researchers (M.C.C. and M.G.Q.H.), and studies selected for inclusion were extracted using a standardized table, including the following information: author, year of publication, country, study design, population studied (sex, age, ethnicity, deleterious habits, sun exposure, symptomatology, and lesion location), clinical features, microscopic features, and malignant transformation rate.
Risk of Bias in the Studies
To avoid measurement bias, the study data were independently extracted by two researchers (M.C.C. and M.G.Q.H.). This information was compared to detect agreements and disagreements and assess whether the extraction was performed well. The risk of selection bias was assessed using the Joanna Briggs Institute’s critical assessment tools [16]. Each question was answered with “yes,” “no,” “not clear,” or “not applicable.”
Two reviewers (M.C.C. and M.G.Q.H.) analyzed the risk of bias separately and classified the articles as “high risk” (when the study reached up to 49% “yes” for the considered parameters), “moderate risk” (50–69% “yes”), and “low risk” (> 70% “yes”) [17]. A conference was held between the two reviewers, and disagreements were resolved by consensus. Graphs were generated using the RevMan 5.4 software (Review Manager 5.4, Cochrane Collaboration).
Analysis of Evidence and Statistics
A database containing the variables and classifications was organized in a Microsoft Office Excel 2016 spreadsheet (Microsoft Corporation, Redmond, WA, USA) to tabulate statistical data. A narrative synthesis was performed, structured around the general information of the population studied, as well as the clinical and microscopic features of AC and malignant transformation rates of the lesion. Quantitative data synthesis, based on percentages, was also performed with data from included studies from random-effects meta-analyses and mixed-effects subgroup analyses performed using Jamovi software, version 1.6.15 (The Jamovi Project 2021, Sydney, NSW, Australia) and the Comprehensive Meta-analysis software (Biostat, Englewood, NJ, USA). In addition, association analyses of clinical features of AC and lip SCC were performed using the Jamovi software, version 1.6.15 (The Jamovi Project 2021, Sydney, NSW, Australia). The chi-square test was used for these nominal qualitative variables, with p < 0.05 considered significant.
Results
Research and Selection of Studies
Initially, 2132 studies were identified in the databases. However, after reading the titles and abstracts, 1097 studies were excluded because they were duplicates, and 955 were considered irrelevant to the topic (κ = 0.90). The remaining 80 studies were eligible for the full-text analysis. Of these, six were not recovered, and 61 were eliminated after applying the inclusion criteria (κ = 0.88). The reasons for the exclusion are detailed in Supplementary Table S3. Finally, 13 studies [2, 18–29] were included in the data extraction. The selection process is summarized in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram (adapted) for new systematic reviews, which included searches of databases, registers, and other sources
Characteristics of the Studies
The general information collected from the included studies is presented in Supplementary Table S4. Twelve [2, 18–20, 22–29] of the 13 studies examined are cross-sectional, whereas one [21] is case–control. The studies were published from 2004 to 2021, with 12 Brazilian studies [2, 19–29] and one Greek [18]. The total sample comprised 728 patients, with a prevalence of AC in men (74.45%, 542 of 728) and a mean age of 57.45 years. Whites were the most impacted (90.69%, 575 of 634), followed by multiracial (7.09%, 45 of 634), black (2.05%, 13 of 634), and Asian (0.15%, 1 of 634). AC was predominantly located on the lower lip (98.50%, 593 of 602), and tobacco use predominated in terms of harmful habits (47.66%, 296 of 621), followed by alcohol consumption (23.64%, 70 of 621). Chronic sun exposure was recorded in 61.69% of the patients (335 of 543). Regarding symptoms, a frequency of 34.29% (143 of 417 patients) was observed, with reports of pain, burning, stinging, and itching (Supplementary Table S4).
Risk of Bias in the Studies
Figure 2 summarizes the risk of bias assessment performed in this study. Regarding cross-sectional studies, ten [2, 18–23, 25–28] had a “low risk” of bias, and two [24, 29] had a “moderate risk” of bias. The case–control study [21] showed a “low risk” of bias.
Fig. 2.
Risk of bias assessed using the JBI appraisal criteria for observational studies. a Cross-sectional studies; b Case–control study
Results of Individual Studies
The variables studied were separated into four categories for better interpretation of the results: clinical features, microscopic features, degree of epithelial dysplasia, and malignant transformation.
Clinical Features
Clinical features were evaluated in all studies [2, 18–29]; however, 12 studies [2, 18–28] provided such information descriptively: dryness (98.56%, 206 of 209), scaling (90.42%, 225 of 282), blurred demarcation between the lip vermilion and skin (84.21%, 288 of 342), atrophy (70.19%, 325 of 463), white spots (68.75%, 55 of 80), fissure (68.18%, 150 of 220), erythema (62.42%, 201 of 322), swelling (53.43%, 101 of 189), white plaque (42.72%, 276 of 646), ulceration (28.68%, 150 of 523), red plaque (25.68%, 47 of 183), and crust (15.81%, 37 of 234) (Supplementary Table S5). Abrantes et al. [29] in turn, developed a clinical categorization based on three criteria: fundamental lesion, lesion color, and lesion surface, with a predominance of fundamental plaque-type lesions (48.33%, 29 of 60), color white (53.33%, 32 of 60), and uneven surface (39.28%, 22 of 56). Supplementary Table S5 provides an overview of the findings.
Microscopic Features
Five studies [2, 19, 24, 26, 28] evaluated microscopic morphological features, the most common being acanthosis (95.62%, 153 of 160), hyperkeratosis (83.50%, 167 of 200), solar elastosis (75.39%, 144 of 191), atrophy (64.42%, 134 of 208), dyskeratosis (63.29%, 50 of 79), inflammatory infiltrate (49.73%, 95 of 191), pleomorphism (45.16%, 14 of 31), hyperchromatism (45.16%, 14 of 31), ulceration (41.98%, 55 of 131), granulosis (37.93%, 11 of 29), epithelial hyperplasia (35.19%, 63 of 179), and vasodilation (21.25%, 34 of 160) (Supplementary Table S5).
Degree of Epithelial Dysplasia
Ten studies [18–22, 25–29] reported the degree of dysplasia according to the World Health Organization (WHO) classification, with mild dysplasia in 34.69% (161 of 464), moderate dysplasia in 26.29% (122 of 464), and severe dysplasia in 16.59% (77 of 464). Three studies [23, 25, 29] also used binary system categorization, with 59.76% (101 of 169) for low risk and 40.23% (68 of 169) for high risk (Supplementary Table S5).
Malignant Transformation
Four studies [2, 18, 20, 24] provided data on malignant transformation of their samples, ranging from 10 [20] to 16.92% [18].
Summary of Results
The clinical features most reported in the studies allowed for the performance of meta-analyses. Dryness (Fig. 3a) was mentioned in four studies [2, 21, 25, 28] and had a prevalence of 99% (95% confidence interval [CI] = 0.98–1.00; heterogeneity: I2 = 0.82%, TAU = 0.002, Q test = 3.834 [p = 0.280]). Scaling (Fig. 3b) was reported in six studies [2, 19, 21, 25, 27, 28] and had a prevalence of 69% (95% CI = 0.52–0.87; heterogeneity: I2 = 92.14%, TAU = 0.206, Q test = 86.309 [p < 0.001]). Blurred demarcation between the lip vermilion and skin (Fig. 3c) was observed in eight studies [2, 19–22, 25, 27, 28] and had a prevalence of 82% (95% CI = 0.66–0.99; heterogeneity: I2 = 98.04%, TAU = 0.231, Q test = 118.536 [p < 0.001]). White plaque (Fig. 3d), in turn, was identified in 12 studies [2, 18–28] and presented a prevalence of 43% (95% CI = 0.34–0.52; heterogeneity: I2 = 79.63%, TAU = 0.130, Q test = 58.898 [p < 0.001]).
Fig. 3.
Forest plots of the first group of clinical characteristics pointed in studies. a Dryness; b Scaling; c Blurred demarcation between the lip vermilion and skin; d White plaque
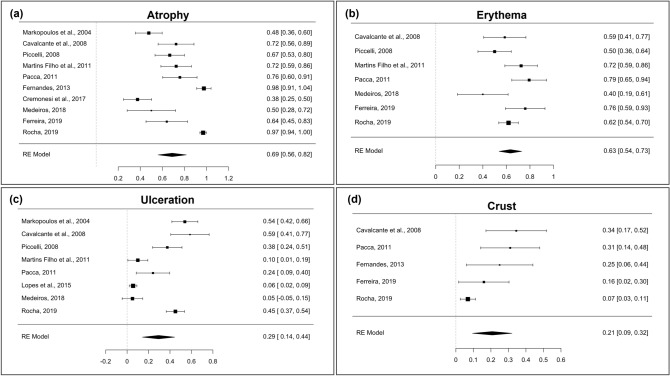
Atrophy (Fig. 4a) was addressed in ten studies [2, 18–22, 24, 25, 27, 28] and presented a prevalence of 69% (95% CI = 0.56–0.82; heterogeneity: I2 = 93.92%, TAU = 0.195, Q test = 184.370 [p < 0.001]). Erythema (Fig. 4b) was identified in seven studies [2, 19–21, 25, 27, 28] and had a prevalence of 63% (95% CI = 0.54–0.73; heterogeneity: I2 = 67.76%, TAU = 0.105, Q test = 16.720 [p = 0.010]). Ulceration (Fig. 4c) was described in eight studies [2, 18–21, 23, 25, 28] and had a prevalence of 29% (95% CI = 0.14–0.44; heterogeneity: I2 = 95.32%, TAU = 0.210, Q test = 153.899 [p < 0.001]). Finally, crust (Fig. 4d) was mentioned in five studies [2, 21, 22, 27, 28] and presented a prevalence of 21% (95% CI = 0.09–0.32; heterogeneity: I2 = 72.84%, TAU = 0.105, Q test = 18.561 [p < 0.001]).
Fig. 4.
Forest plots of the second group of clinical characteristics pointed in studies. a Atrophy; b Erythema; c Ulceration; d Crust
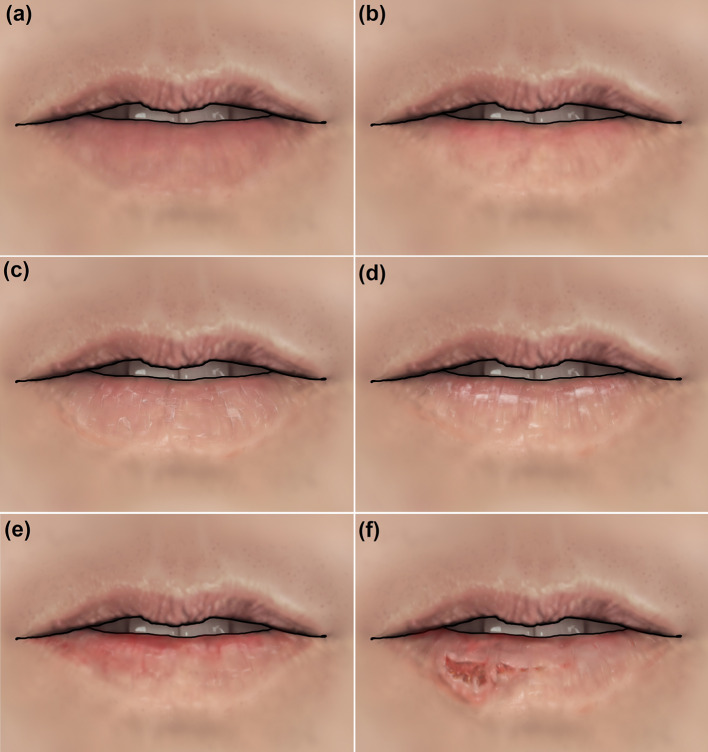
Figure 5 was drawn based on the findings of the primary studies of this investigation and aimed to help dermatologists, dentists, and other health professionals identify these clinical aspects more easily.
Fig. 5.
Illustrations simulating the main clinical characteristics identified. a Normal lip; b Blurred demarcation between the lip vermilion and skin; c Scaling and dryness; d White areas; e Red areas; f Ulcerations, crusts, and bleeding
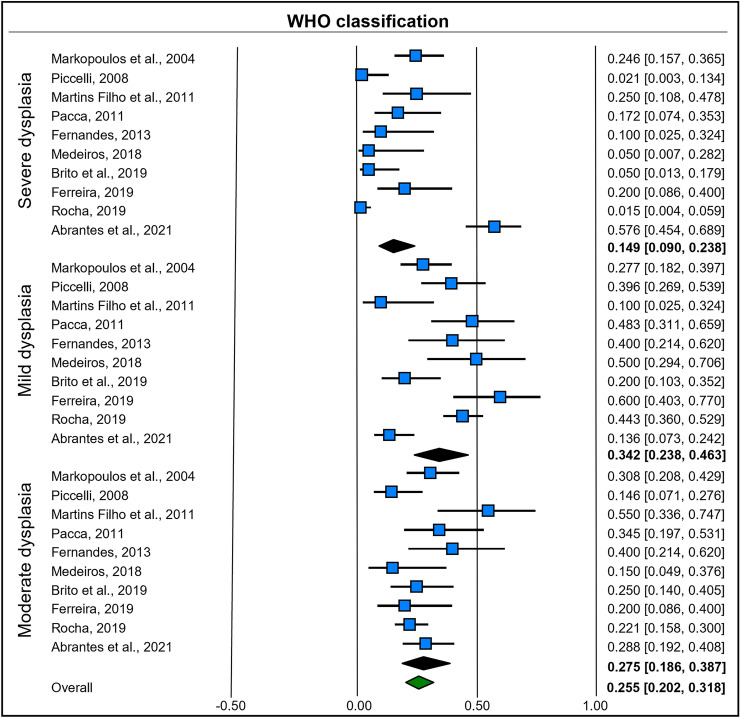
The degree of epithelial dysplasia identified in the studies included in the systematic review enabled the construction of subgroup analyses based on mixed effects. A random-effects model was used to combine studies within each subgroup, and a fixed effect was used to assess the overall effect of each subgroup. Study-to-study variance was considered the same for all subgroups, with a value calculated within the subgroups and then pooled among them.
Ten studies [18–22, 25–29] indicated the degree of epithelial dysplasia based on the WHO classification. Mild dysplasia had a prevalence of 34.2% (95% CI = 0.23–0.46; heterogeneity: I2 = 75.57%, TAU = 0.593, Q test = 36.852 [p < 0.001]). In contrast, moderate dysplasia had a prevalence of 27.5% (95% CI = 0.18–0.38; heterogeneity: I2 = 48.14%, TAU = 0.337, Q test = 17.354 [p = 0.043]). Finally, severe dysplasia had a prevalence of 14.9% (95% CI = 0.09–0.23; heterogeneity: I2 = 87.25%, TAU = 1.275, Q test = 70.632 [p < 0.001]) (Fig. 6).
Fig. 6.
Forest plot of subgroup analysis based on mixed effects for the WHO classification
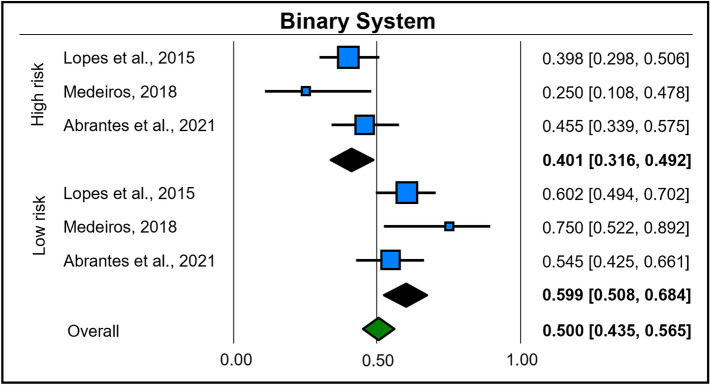
Three studies [23, 25, 29] also addressed the classification of degrees of epithelial dysplasia from the binary system. Lesions considered to be of low risk had a prevalence of 59.9% (95% CI = 0.50–0.68; heterogeneity: I2 = 23.08%, TAU = 0.161, Q test = 2.600 [p = 0.272]), and those considered high risk had a prevalence of 40.1% (95% CI = 0.31–0.49; heterogeneity: I2 = 23.08%, TAU = 0.161, Q test = 2.600 [p = 0.272]) (Fig. 7).
Fig. 7.
Forest plot of subgroup analysis based on mixed effects for the Binary System classification
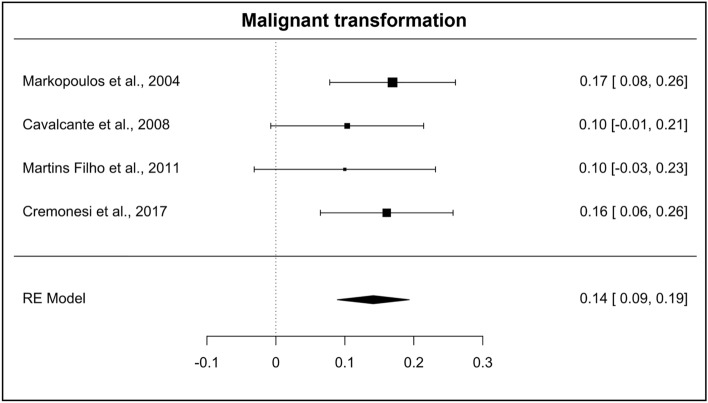
Four studies [2, 18, 20, 24] also provided information on the malignant transformation of AC, enabling the development of a meta-analysis of these results. It was evidenced that the malignant transformation of AC had a prevalence of 14% (95% CI = 0.09–0.19; heterogeneity: I2 = 0%, TAU = 0.000, Q test = 1.344 [p = 0.719]) (Fig. 8).
Fig. 8.
Forest plot on the malignant transformation rate
Missing Data from the Included Studies
Regarding the general information collected, one study [2] did not provide information on the mean age of the population, whereas another study [24] did not provide any information on ethnicity. Two studies [27, 29] did not provide information regarding deleterious habits. Five studies [2, 19, 21, 26, 27] did not provide information regarding sun exposure, and symptoms were not addressed in five studies either [18, 24, 25, 27, 29]. Four studies [2, 19, 21, 25] did not describe the lesion sites.
Regarding the microscopic features, some studies [18, 20–23, 25, 27, 29] reported only the degrees of epithelial dysplasia, whereas others [2, 24] reported only the morphological microscopic features.
Publication Bias
No publication bias analysis was performed in this research, as this type of analysis does not apply to prevalence data, as there is no reason to assume that a survey with high or low prevalence will have a higher or lower probability of publication, following the guidance of Borenstein [30].
Additional Analyses
Ferreira [27] and Rocha [28] identified clinical features of AC and lip SCC, allowing the performance of tests of association of these aspects between the diseases, to determine which features would be more aggressive or more linked to malignant lesions. The chi-square test was used, with p < 0.05 considered significant. Regarding the clinical features, statistically significant results were noted for crust (p < 0.001), erythema (p < 0.001), scaling (p = 0.001), and ulceration (p < 0.001).
Regarding the crust, a lower occurrence was observed in patients with AC (8.3%) than that in patients with lip SCC (43.7%), suggesting that this feature is possibly related to more aggressive and malignant lesions. Regarding erythema, the analysis showed that patients with lip SCC were more likely to have this feature (95.4%) than those with AC (64.1%). Regarding scaling, a higher prevalence was noted in individuals with AC (87.2%) than in those with lip SCC (70.1%). Finally, a strong prevalence of ulceration was observed in patients with lip SCC (88.2%) compared with those with AC (45%).
The following clinical features were not statistically significant (p > 0.05): blurred demarcation between the lip vermilion and skin, erosion, fissures, and white plaques (Table 1).
Table 1.
Association of clinical and microscopic features of actinic cheilitis and lip squamous cell carcinoma
| Lip SCC N (%) | AC N (%) | P value | ||
|---|---|---|---|---|
| Clinical Features | ||||
| Blurred demarcation between the lip vermilion and skin | No | 1 (1.1%) | 1 (0.6%) | 0.674 |
| Yes | 86 (98.9%) | 155 (99.4%) | ||
| Crust | No | 49 (56.3%) | 143 (91.7%) | < 0.001* |
| Yes | 38 (43.7%) | 13 (8.3%) | ||
| Erosion | No | 5 (5.7%) | 13 (8.3%) | 0.461 |
| Yes | 82 (94.3%) | 143 (91.7%) | ||
| Erythema | No | 4 (4.6%) | 56 (35.9%) | < 0.001* |
| Yes | 83 (95.4%) | 100 (64.1%) | ||
| Fissure | No | 3 (5.9%) | 7 (5.3%) | 0.886 |
| Yes | 48 (94.1%) | 124 (94.7%) | ||
| Scaling | No | 26 (29.9%) | 20 (12.8%) | 0.001* |
| Yes | 61 (70.1%) | 136 (87.2%) | ||
| Ulceration | No | 6 (11.8%) | 72 (55.0%) | < 0.001* |
| Yes | 45 (88.2%) | 59 (45.0%) | ||
| White plaque | No | 28 (32.2%) | 66 (42.3%) | 0.120 |
| Yes | 59 (67.8%) | 90 (57.7%) | ||
| Microscopic features | ||||
| Acanthosis | No | 3 (5.9%) | 3 (2.3%) | 0.223 |
| Yes | 48 (94.1%) | 128 (97.7%) | ||
| Atrophy | No | 14 (27.5%) | 17 (13.0%) | 0.020* |
| Yes | 37 (72.5%) | 114 (87.0%) | ||
| Epithelial hyperplasia | No | 40 (78.4%) | 109 (83.2%) | 0.453 |
| Yes | 11 (21.6%) | 22 (16.8%) | ||
| Inflammatory infiltrate | No | 3 (5.9%) | 88 (67.2%) | < 0.001* |
| Yes | 48 (94.1%) | 43 (32.8%) | ||
| Solar elastosis | No | 16 (31.4%) | 31 (23.7%) | 0.286 |
| Yes | 35 (68.6%) | 100 (76.3%) | ||
| Ulceration | No | 2 (3.9%) | 76 (58.0%) | < 0.001* |
| Yes | 49 (96.1%) | 55 (42.0%) | ||
| Vasodilation | No | 11 (21.6%) | 126 (96.2%) | < 0.001* |
| Yes | 40 (78.4%) | 5 (3.8%) | ||
SCC squamous cell carcinoma, AC actinic cheilitis
*Statistically significant difference
Ferreira [27] and Rocha [28] also pointed out the microscopic features of AC and lip SCC, allowing these findings to be associated as well. Regarding microscopic features, a statistically significant result was noted for atrophy (p = 0.020), inflammatory infiltrate (p < 0.001), ulceration (p < 0.001), and vasodilation (p < 0.001).
Individuals with AC had a higher frequency of atrophy (87%) than those with lip SCC (72.5%). Regarding the inflammatory infiltrate, a strong prevalence was noted in patients with lip SCC (94.1%), whereas in patients with AC, this prevalence was much lower (32.8%). There was also a strong prevalence of ulceration in patients with lip SCC (96.1%), unlike that observed in patients with AC (42%). Vasodilation was also more prevalent in patients with lip SCC (78.4%) than in those with AC (3.8%). These results suggest that these microscopic features are likely to be associated with aggressive and malignant lesions.
The following microscopic features were not statistically significant (p > 0.05): acanthosis, epithelial hyperplasia, and solar elastosis (Table 1).
Discussion
The prevalence of AC described in the literature varies from 0.9 to 43.24% [31]. According to the data obtained in this study, the profile of patients with this disease is as follows: male (74.45%), white (90.69%), with a mean age of 57.45 years and regular exposure to UV radiation (61.69%). These findings are consistent with previous research [7, 13, 32].
According to Melo et al. [32], approximately 82% of cases of AC and 80% of lip SCC affect men; 85% of individuals with AC and 87% of those with lip SCC are fair-skinned individuals. Due to the lower amount of melanin in the skin, fair-skinned individuals are more susceptible to the development of diseases whose etiologic factor is UV radiation [2, 32–34]. White individuals are 10.8 times more likely to be affected by AC, according to Moreira et al. [35].
In addition to skin color, age has been identified as a significant factor in the development of AC. When considering the UV radiation accumulated over time, the relationship between increasing age and the presence of the disease tends to be proportional, especially in fair-skinned individuals [20, 35].
Moreover, most studies included in this systematic review were Brazilian. Tropical countries, such as Brazil, where several workers perform their activities outdoors or are used for prolonged exposure to UV radiation, have a greater propensity to develop these lesions, mainly owing to the lack of use of protective agents, such as sunscreen, and barriers, such as caps and hats [34, 36, 37].
Regarding deleterious habits, the results revealed a relationship between tobacco use and the development of lesions, as more than 47% of individuals reported being smokers or ex-smokers. Although several tobacco substances have been linked to the development of intraoral cancer, their role in the pathophysiology of AC and lip SCC is unknown. Some studies suggest that smoking is an important secondary factor in lip SCC, affecting both the upper and lower lips, as the development of lesions has been reported at the site where tobacco is placed [32]. However, more research is needed to clarify the potential contribution of tobacco consumption to the etiopathogenesis of AC and lip SCC [32].
Regarding the location of the lesion, the literature reveals that most lesions are found in the lower lip [13, 31, 32, 38], corroborating the present study, which found that the lower lip was affected in 98.50% of the cases. Several authors have characterized AC as restricted to the lower lip; however, there are some cases of AC in the upper lip reported in the literature [18, 29, 32]. The upper lip was impacted in nine cases (1.49%) of the patients reported in this review.
In terms of symptoms, early AC lesions often go unnoticed, as they are asymptomatic in most patients [33, 36, 39]. The frequency of symptoms was observed in 34.29% of the patients in the present investigation, with reports of pain, burning, stinging, and itching. Generally, early lesions tend to be asymptomatic; consequently, the professional must understand that the absence of symptoms is relevant for early diagnosis [34].
Clinically, AC has broad features, including white lesions commonly associated with scaling and dryness [2, 33, 36]. Atrophic zones are also apparent and are often accompanied by smooth and blotchy areas, as well as fissures. Other typical clinical signs are alteration of the vermilion of the lips and blurred demarcation between the lip vermilion and skin [2, 33, 40]. This corroborates the findings of this review, which found that most studies point to these features as being the most prevalent in patients with AC. Among the most common, dryness (99%; 95% CI = 0.98–1.00), blurred demarcation between the lip vermilion and skin (82%; 95% CI = 0.66–0.99), atrophy (69%; 95% CI = 0.56–0.82), and scaling (69%; 95% CI = 0.52–0.87) led to the prevalence ranking.
Conversely, crusts and ulcerations are visible in more advanced cases and are less common [2, 33, 40]. This is supported by the results of the current investigation, which detected crusts in only 21% (95% CI = 0.09–0.32) and ulcerations in 29% (95% CI = 0.14–0.44) of cases. Another interesting finding when comparing the clinical features of AC and lip SCC is that the appearance of crusts, ulcerations, and erythematous areas is more prevalent in lip SCC than that in AC (p < 0.001), whereas scaling was more evident in AC lesions than that in lip SCC (p < 0.001). The literature states that uniform white lesions are associated with lower levels of epithelial dysplasia, whereas mixed and red lesions are associated with higher levels of dysplasia [7, 29, 38].
As AC has different clinical features, microscopic aspects can be useful for closing the diagnosis and determining the stage of the disease [23, 36, 41]. Previous research has shown that the lesion is microscopically characterized by solar elastosis and varying degrees of inflammatory infiltrate [13, 36, 41]. The epithelium is often atrophic or acanthotic, with thickening of the keratin layer and varying degrees of epithelial dysplasia [41]. Acanthosis was the most prevalent microscopic change seen in the studies included in this analysis (95.62%), whereas atrophic epithelium was found in 64.42%. Furthermore, 75.39% of the patients had solar elastosis.
The studies in this review that used the WHO classification [18–22, 25–29] showed a higher prevalence of lesions with mild dysplasia (34.2%; 95% CI = 0.23–0.46), followed by moderate dysplasia (27.5%; 95% CI = 0.18–0.38), and severe dysplasia (14.9%; 95% CI = 0.09–0.23). In addition, three studies [23, 25, 29] used the binary system and found a prevalence of 59.9% (95% CI = 0.50–0.68) and 40.1% (95% CI = 0.31–0.49) for low and high risks, respectively. However, owing to the non-uniformity of the dysplasia criteria/classification among the different evaluators of the studies, as well as the fact that these gradations have changed over time, both results of the microscopic classification systems (WHO and binary system) should be interpreted with caution.
Importantly, meta-analyses have revealed high rates of heterogeneity among studies in most clinical features and degrees of dysplasia. These prevalence disparities may be related to the professionals’ difficulty in identifying their numerous clinical features, as well as the different criteria for microscopic diagnosis.
The WHO classification method to assess the degrees of dysplasia is the most used and currently recommended [38]. It is based on morphological criteria (cytological and architectural), defining dysplasia as mild, moderate, and severe [38, 42]. However, consistency in classifying these conditions is a challenge, and even when there is agreement among examiners, these categorization methods are extremely subjective [34, 43]. It is particularly difficult in the case of moderate dysplasia and, consequently, there are no criteria to precisely define the degrees of dysplasia [13]. Kujan et al. [44] proposed a binary system to categorize dysplasias into low and high risk of malignant transformation. Consequently, having only two groups is expected to help pathologists reduce subjectivity [13].
According to Kujan et al. [44], the binary system with four architectural and five cytological features showed higher inter-examiner agreement (κ = 0.50) than the WHO system (κ = 0.22). When compared with the WHO three-tier system, Nankivell et al. [45] also stated that the binary system has greater reproducibility and similar prognostic ability. In fact, when analyzing the heterogeneity data of the binary system of the present meta-analyses, more homogeneous data in both degrees were observed (I2 = 23.08%, TAU = 0.161, Q test = 2.600 [p = 0.272]) than those obtained by the WHO classification (mild dysplasia: I2 = 75.57%, TAU = 0.593, Q test = 36.852 [p < 0.001]; moderate dysplasia: I2 = 48.14%, TAU = 0.337, Q test = 17.354 [p = 0.043]; and severe dysplasia: I2 = 87.25%, TAU = 1.275, Q test = 70.632 [p < 0.001]).
However, according to Cavalcante et al. [46], the WHO classification system is more reliable than the binary system, as it considers the incidence of cytological and architectural changes throughout the mucosa, whereas the binary system only quantifies the changes regardless of the level of mucosal involvement caused by dysplastic changes. Therefore, we believe that the classification proposed by the WHO is more reliable than the binary system. However, for both methods, there is evidence that adequate training and consensus reporting by at least two pathologists can increase the accuracy and reliability of dysplasia classifications [38, 44, 47].
The available literature on the rate of malignant transformation of AC is controversial, ranging from 10 to 30% [3, 9, 10]. However, a systematic review performed by Dancyger et al. [33] showed a rate of 3.07%. In contrast, the present meta-analysis found a prevalence of 14% (95% CI = 0.09–0.19).
In terms of malignant transformation and its relationship with the degree of dysplasia, Nagata et al. [48] stated that the transition from AC to lip SCC cannot be solely predicted by the degree of dysplasia, since they found varying degrees of dysplasia when examining epithelial dysplasia next to lip malignancies. In contrast, Pilati et al. [49] compared each criterion of epithelial dysplasia in AC and lip SCC in order to determine whether any of them, by itself, may be more significant than the others and whether there are criteria that could suggest a more severe disease. In the comparison of the WHO dysplasia classification and the epithelial dysplasia criterion, drop-shaped projections were seen in all of the examined lip carcinomas, associated with hyperplasia of basal layer and irregular epithelial stratification, and they were considerably bigger with increasing dysplasia. Additionally, moderate dysplasia, severe dysplasia, and lip SCC all had a prevalence of dyskeratosis and keratin pearls [49].
Furthermore, Garcia et al. [50] investigated the relationship between cytokeratins CK10 and CK13 expression and the cell proliferation index of lip SCC and AC with varying degrees of dysplasia. As a result, AC lesions showed immunopositivity for CK10 and CK13, with decreased or nonexistent expression in dysplastic areas, whereas lip SCC showed varied CK13 expression and immunonegativity for CK10. These findings demonstrate that CK10 expression is lower in dysplastic areas and that the cell proliferation index is directly related to the degree of epithelial dysplasia [50]. Therefore, these parameters should be examined further in the identification of dysplasias in AC, as they may aid in determining disease progression and severity.
Despite this, owing to the anatomical position and clinical behavior of the lesion, individuals who develop lip SCC have a higher survival rate than those with other head and neck malignancies [51]. Furthermore, the 5-year survival rate for patients with lip SCC ranges from 62 to 79% [51].
Overall, little is known about the prevalence of the numerous clinicopathological features of AC, reiterating the need to conduct this systematic review. However, it is important to emphasize that this study had some limitations. First, due to the fact of evaluating cross-sectional observational and case–control studies, primary studies may be susceptible to possible errors and confounding factors. Furthermore, no uniformity in the criteria and classification of the clinical and microscopic features of the lesions among the potential examiners of these patients was noted, which may lead to limitations in the interpretation of the results. Another limitation of this review and previous investigations on AC is the absence of reliable clinical criteria to determine its clinical features.
This study revealed several features of AC, providing an overview of the disease. However, further research on the clinical, demographic, and microscopic parameters of AC should be conducted because there are limited data in the literature on this subject. It would also be interesting to create policy guides for the standardization of clinical criteria so that future systematic reviews present more robust and homogeneous results on the subject.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Conceptualization: MCC, PSdSS, CMFR; Methodology: MCC, MGQH, MPM; Formal analysis and investigation: MCC, MGQH, HMH, CMFR; Writing—original draft preparation: MCC, HMH, IRFR, CMFR; Writing—review and editing: MCC, MGQH, MPM, HMH, PSdSS, IRFR, CMFR; Funding acquisition: MCC; Supervision: CMFR.
Funding
The main author (Mailon Cury Carneiro) has received a master’s scholarship from the National Council for Scientific and Technological Development (CNPq), Brazil. (Grant Number: 133375/2020–0).
Data Availability
All data and material are available upon reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
Informed Consent
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jadotte YT, Schwartz RA. Solar cheilosis: an ominous precursor. J Am Acad Dermatol. 2012;66:173–184. doi: 10.1016/j.jaad.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 2.Cavalcante AS, Anbinder AL, Carvalho YR. Actinic cheilitis: clinical and histological features. J Oral Maxillofac Surg. 2008;66:498–503. doi: 10.1016/j.joms.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Piñera-Marques K, Lorenço SV, da Silva LF, Sotto MN, Carneiro PC. Actinic lesions in fishermen’s lower lip: clinical, cytopathological and histopathologic analysis. Clinics. 2010;65:363–367. doi: 10.1590/S1807-59322010000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peris K, Micantonio T, Piccolo D, Concetta M. Dermoscopic features of actinic keratosis. J Dtsch Dermatol Ges. 2007;5:970–975. doi: 10.1111/j.1610-0387.2007.06318.x. [DOI] [PubMed] [Google Scholar]
- 5.Lucena EE, Costa DC, Da Silveira EJ, Lima KC. Prevalence and factors associated to actinic cheilitis in beach workers. Oral Dis. 2012;18:575–579. doi: 10.1111/j.1601-0825.2012.01910.x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzaga AK, de Oliveira PT, da Silveira ÉJ, Queiroz LM, de Medeiros AM. Diclofenac sodium gel therapy as an alternative to actinic cheilitis. Clin Oral Investig. 2018;22:1319–1325. doi: 10.1007/s00784-017-2237-5. [DOI] [PubMed] [Google Scholar]
- 7.Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:582–590. doi: 10.1016/j.oooo.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 8.Luna-Ortiz K, Güemes-Meza A, Villavicencio-Valencia V, Mosqueda-Taylor A. Lip cancer experience in Mexico. An 11-year retrospective study. Oral Oncol. 2004;40:992–999. doi: 10.1016/j.oraloncology.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Ntomouchtsis A, Karakinaris G, Poulolpoulos A, Kechagias N, Kittikidou K, Tsompanidou C, et al. Benign lip lesions: a 10-year retrospective study. Oral Maxillofac Surg. 2010;14:115–118. doi: 10.1007/s10006-009-0196-y. [DOI] [PubMed] [Google Scholar]
- 10.Chaves YN, Torezan LA, Lourenço SV, Neto CF. Evaluation of the efficacy of photodynamic therapy for the treatment of actinic cheilitis. Photodermatol Photoimmunol Photomed. 2017;33:14–21. doi: 10.1111/phpp.12281. [DOI] [PubMed] [Google Scholar]
- 11.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:139–148. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Huber MA. White oral lesions, actinic cheilitis, and leukoplakia: confusions in terminology and definition: facts and controversies. Clin Dermatol. 2010 doi: 10.1016/j.clindermatol.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Câmara PR, Dutra SN, Júnior TA, Fontes KB, Azevedo RS. A comparative study using WHO and binary oral epithelial dysplasia grading systems in actinic cheilitis. Oral Dis. 2016;22:523–529. doi: 10.1111/odi.12484. [DOI] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016 doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, et al., editors. JBI Manual for Evidence Synthesis. Adelaide: JBI; 2020. [Google Scholar]
- 17.Polmann H, Domingos FL, Melo G, et al. Association between sleep bruxism and anxiety symptoms in adults: a systematic review. J Oral Rehabil. 2019 doi: 10.1111/joor.12785. [DOI] [PubMed] [Google Scholar]
- 18.Markopoulos A, Albanidou-Farmaki E, Kayavis I. Actinic cheilitis: clinical and pathologic characteristics in 65 cases. Oral Dis. 2004;10:212–216. doi: 10.1111/j.1601-0825.2004.01004.x. [DOI] [PubMed] [Google Scholar]
- 19.Piccelli HR (2008) Avaliação clínica, histopatológica e imuno-histoquímica de 48 casos de queilite actínica. Master’s Thesis, Universidade Católica de Goiás
- 20.Martins Filho PR, Da Silva LC, Piva MR. The prevalence of actinic cheilitis in farmers in a semi-arid northeastern region of Brazil. Int J Dermatol. 2011;50:1109–1114. doi: 10.1111/j.1365-4632.2010.04802.x. [DOI] [PubMed] [Google Scholar]
- 21.Pacca FO, Marcucci G, Nunes FD, Silva CE, Cerri A. Aspectos clínicos e histológicos en la queilitis actínica crónica, su relación con el Virus del Papiloma Humano. Odontoestomatologia. 2011;13:45–53. [Google Scholar]
- 22.Fernandes LG (2013) Caracterização clínico-histopatológica e avaliação terapêutica de fotoprotetor nas queilites actínicas. Master’s Thesis, Faculdade de Odontologia da Universidade de São Paulo
- 23.Lopes ML, Silva Júnior FL, Lima KC, Oliveira PT, Silveira ÉJ. Clinicopathological profile and management of 161 cases of actinic cheilitis. An Bras Dermatol. 2015;90:505–512. doi: 10.1590/abd1806-4841.20153848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cremonesi AL, Quispe RA, Garcia AS, Santos OS. Queilite actínica: um estudo retrospectivo das características clinicas e histopatológicas. Arq Med Hosp Fac Cienc Med St Casa São Paulo. 2017;62:7–11. [Google Scholar]
- 25.Medeiros CK (2018) Queilite Actínica: índice de análise clínica. Master’s Thesis, Universidade Federal do Rio Grande do Norte
- 26.Brito LN, Bonfim AC, Gomes DQ, Alves MP, Nonaka CF, Godoy GP. Clinical and histopathological study of actinic cheilitis. Rev Odontol da UNESP. 2019;48:e20190005. doi: 10.1590/1807-2577.00519. [DOI] [Google Scholar]
- 27.Ferreira OS (2019) Uso da microscopia confocal de reflectância na avaliação de queilite actínica: padrões pareados com histologia e sinais precoces de evolução para carcinoma espinocelular. Dissertation, Faculdade de Medicina da Universidade de São Paulo
- 28.Rocha AF (2019) Caracterização da queilite actínica como desordem potencialmente maligna oral. Master’s Thesis, Faculdade de Odontologia da Universidade Estadual Paulista
- 29.de Castro AT, Fonsêca TC, Cabral MG, Agostini M, Benevenuto de Andrade BA, Romañach MJ, et al. Epithelial dysplasia in actinic cheilitis: microscopic study of 70 cases from Brazil. Head Neck Pathol. 2021;15:566–571. doi: 10.1007/s12105-020-01250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borenstein M. Common mistakes in meta-analysis and how to avoid them. Englewood: Biostat Inc; 2019. [Google Scholar]
- 31.Rodríguez-Blanco I, Flórez Á, Paredes-Suárez C, Rodríguez-Lojo R, González-Vilas D, Ramírez-Santos A, et al. Actinic cheilitis prevalence and risk factors: a cross-sectional, multicentre study in a population aged 45 Years and over in North-West Spain. Acta Derm Venereol. 2018;98:970–974. doi: 10.2340/00015555-3014. [DOI] [PubMed] [Google Scholar]
- 32.Mello FW, Melo G, Modolo F, Rivero ER. Actinic cheilitis and lip squamous cell carcinoma: literature review and new data from Brazil. J Clin Exp Dent. 2019;11:62–69. doi: 10.4317/jced.55133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dancyger A, Heard V, Huang B, Suley C, Tang D, Ariyawardana A. Malignant transformation of actinic cheilitis: a systematic review of observational studies. J Invest Clin Dent. 2018;9:e12343. doi: 10.1111/jicd.12343. [DOI] [PubMed] [Google Scholar]
- 34.Silva LV, de Arruda JA, Abreu LG, Ferreira RC, da Silva LP, Pelissari C, et al. Demographic and clinicopathologic features of actinic cheilitis and lip squamous cell carcinoma: a Brazilian multicentre study. Head Neck Pathol. 2020;14:899–908. doi: 10.1007/s12105-020-01142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira P, Assaf AV, Cortellazzi KL, Junior TA, Azevedo RS. Social and behavioural associated factors of actinic cheilitis in rural workers. Oral Dis. 2021;27:911–918. doi: 10.1111/odi.13610. [DOI] [PubMed] [Google Scholar]
- 36.Sarmento DJ, da Costa Miguel MC, Queiroz LM, Godoy GP, Da Silveira ÉJ. Actinic cheilitis: clinicopathologic profile and association with degree of dysplasia. Int J Dermatol. 2014;53:466–472. doi: 10.1111/ijd.12332. [DOI] [PubMed] [Google Scholar]
- 37.Corrêa MD. Solar ultraviolet radiation: properties, characteristics and amounts observed in Brazil and South America. An Bras Dermatol. 2015;90:297–313. doi: 10.1590/abd1806-4841.20154089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon: IARC Press; 2017. [Google Scholar]
- 39.Vieira RA, Minicucci EM, Marques ME, Marques SA. Actinic cheilitis and squamous cell carcinoma of the lip: clinical, histopathological and immunogenetic aspects. An Bras Dermatol. 2012;87:105–114. doi: 10.1590/S0365-05962012000100013. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira A, Ribeiro LC, da Silva F, Martins-Filho PRS. Prevalence of and risk factors for actinic cheilitis in Brazilian fishermen and women. Int J Dermatol. 2014;53:1370–1376. doi: 10.1111/ijd.12526. [DOI] [PubMed] [Google Scholar]
- 41.Gonzaga AK, Mafra RP, da Silva LP, de Almeida FR, de Souza LB, Pinto LP. Actinic cheilitis: morphometric parameters and its relationship with the degree of epithelial dysplasia. Acta Histochem. 2020;122:151452. doi: 10.1016/j.acthis.2019.151452. [DOI] [PubMed] [Google Scholar]
- 42.Müller S. Update from the 4th edition of the World Health organization of head and neck tumours: tumours of the oral cavity and mobile tongue. Head Neck Pathol. 2017;11:33–40. doi: 10.1007/s12105-017-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranganathan K, Kavitha L. Oral epithelial dysplasia: Classifications and clinical relevance in risk assessment of oral potentially malignant disorders. J Oral Maxillofac Pathol. 2019;23:19–27. doi: 10.4103/jomfp.JOMFP_13_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42:987–993. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Nankivell P, Williams H, Matthews P, Suortamo S, Snead D, McConkey C, et al. The binary oral dysplasia grading system: Validity testing and suggested improvement. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:87–94. doi: 10.1016/j.oooo.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Cavalcante DR, de Oliveira PS, Góis SM, Soares AF, Cardoso JC, Padilha FF, et al. Effect of green propolis on oral epithelial dysplasia in rats. Braz J Otorhinolaryngol. 2011;77:278–284. doi: 10.1590/S1808-86942011000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Speight PM, Khurram SA, Kujan O. Oral potentially malignant disorders: risk of progression to malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018;125:612–627. doi: 10.1016/j.oooo.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Nagata G, Santana T, Queiroz A, Caramez RH, Trierveiler M. Evaluation of epithelial dysplasia adjacent to lip squamous cell carcinoma indicates that the degree of dysplasia is not associated with the occurrence of invasive carcinoma in this site. J Cutan Pathol. 2018;45:647–651. doi: 10.1111/cup.13270. [DOI] [PubMed] [Google Scholar]
- 49.Pilati S, Bianco BC, Vieira D, Modolo F. Histopathologic features in actinic cheilitis by the comparison of grading dysplasia systems. Oral Dis. 2017;23:219–224. doi: 10.1111/odi.12597. [DOI] [PubMed] [Google Scholar]
- 50.Garcia NG, Oliveira DT, Lauris JR, Domingues MA, Minicucci EM, Soares CT. Loss of cytokeratin 10 indicates malignant transformation in actinic cheilitis. Clin Oral Investig. 2016;20:745–752. doi: 10.1007/s00784-015-1557-6. [DOI] [PubMed] [Google Scholar]
- 51.Guntinas-Lichius O, Wendt T, Buentzel J, Esser D, Lochner P, Mueller A, et al. Head and neck cancer in Germany: a site-specific analysis of survival of the Thuringian cancer registration database. J Cancer Res Clin Oncol. 2010;136:55–63. doi: 10.1007/s00432-009-0636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material are available upon reasonable request.