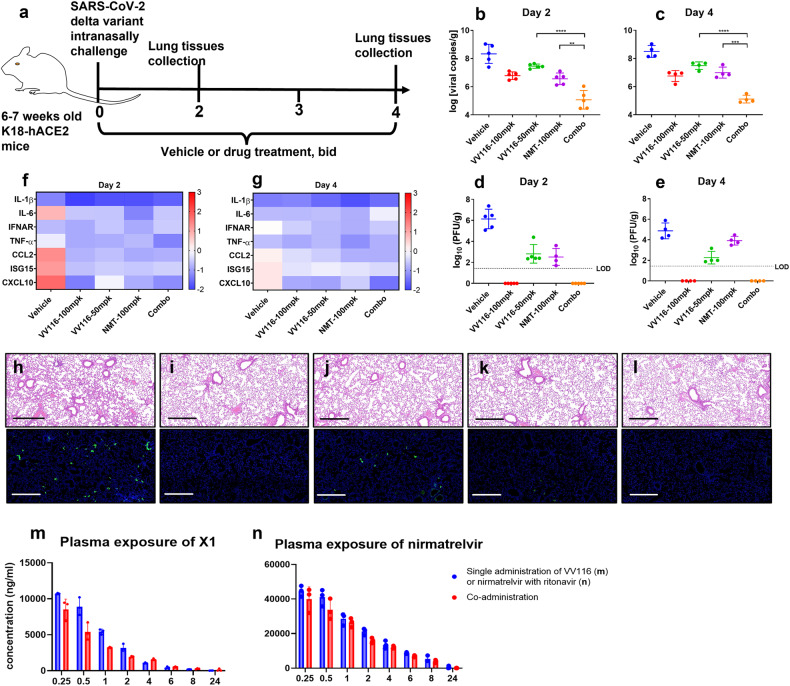
Fig. 4.
In vivo efficacy of VV116, nirmatrelvir, and the VV116 plus nirmatrelvir combination in K18-hACE2 mice infected with the SARS-CoV-2 Delta variant. a Schematic of the experimental design for therapeutic treatment in K18-hACE2 mice. The mice were intranasally challenged with 1000 pfu of the SARS-CoV-2 Delta variant. The mice were divided into five groups (n = 9 for each group): the vehicle group, the group receiving VV116 100 mpk, the group receiving VV116 50 mpk, the group receiving nirmatrelvir 100 mpk with ritonavir 50 mpk, and the group receiving the drug combination (Combo) of VV116 50 mpk and nirmatrelvir 100 mpk with ritonavir 50 mpk. The vehicle or drugs were orally administered at 2 h post-infection and were then administered bis in die (b.i.d.) at 8-h intervals from day 1 to day 4. Lung tissues were collected at 2 days post-infection (n = 5) and 4 days post-infection (n = 4). b and c Determination of viral RNA copies targeting the gene of receptor binding domain in the lungs collected at day 2 and day 4 by real-time fluorescence quantitative PCR. d and e Determination of viral titers in lungs collected at day 2 and day 4 by plaque assay. f and g Cytokine gene expression was measured in the lungs at days 2 and 4. The relative gene expression of IL-1β, IL-6, IFNAR, TNF-α, CCL2, CXCL10, and ISG15 was compared to that of unchallenged mice. h–l Histopathological analysis (hematoxylin-eosin) and immunofluorescence staining to detect the SARS-CoV-2 antigen in lung tissues of the vehicle (h), VV116 100 mpk (i), VV116 50 mpk (j), nirmatrelvir 100 mpk with ritonavir 50 mpk (k), and drug combination (Combo) VV116 50 mpk and nirmatrelvir 100 mpk with ritonavir 50 mpk (l) groups. Scale bars indicate 500 µm. m and n Plasma pharmacokinetic analysis of C57BL/6 J mice that received oral doses of VV116 (single administration), nirmatrelvir + ritonavir (single administration), and VV116 + nirmatrelvir + ritonavir (co-administration) at 50, 100 + 50, and 50 + 100 + 50 mg/kg, respectively. Data on the viral copies and viral titers were statistically analyzed with Student’s t-test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; and ns, not significant