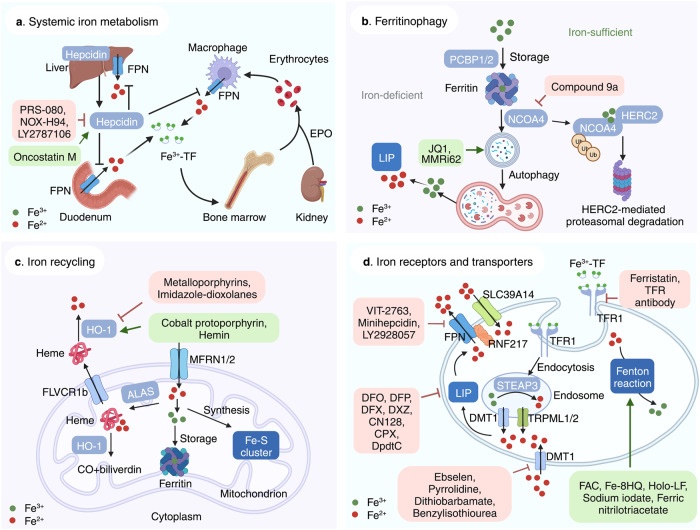
Fig. 3.
Targeting ferroptosis in iron metabolism. a Overview of systemic iron homeostasis. Labile iron binds to transferrin (TF) in the blood, and senescent erythrocytes are phagocytized by macrophages, releasing iron ions back into the circulation. The primary regulatory mechanism of iron homeostasis involves liver-derived hepcidin, which controls the cellular export of iron via ferroportin (FPN). b Overview of the various processes that involve ferritin under iron-deficient and iron-sufficient conditions. When cellular iron is sufficient, ferritin stores iron. Under iron-deficient conditions, ferritin undergoes NCOA4-mediated ferritinophagy and releases iron. c The active labile iron pool can be used either directly for incorporation into iron-containing proteins or transported into the mitochondria. d Overview of iron transporters in the plasma membrane and in lysosomes. Molecules in the pink and green text boxes are inhibitors or activators, respectively, of the pathways that regulate iron metabolism and suppress or trigger, respectively, ferroptosis. ALAS 5-amibolevulinic acid synthase, DMT1 proton-coupled divalent metal ion transporter 1, EPO erythropoietin; FLVCR1b, FLVCR heme transporter 1b, HERC2 HECT and RLD domain containing E3 ubiquitin protein ligase 2, HO-1 heme oxygenase 1, LIP labile iron pool, MFRN mitoferrin, NCOA4 nuclear receptor coactivator 4, PCBP poly(rC)-binding protein, RNF217 E3 ubiquitin protein ligase RNF217, SLC solute carrier family, STEAP 6-transmembrane epithelial antigen of the prostate metalloreductase family, TFR1 transferrin receptor protein 1, TRPML lysosomal cation channel mucolipin. Created with BioRender.com