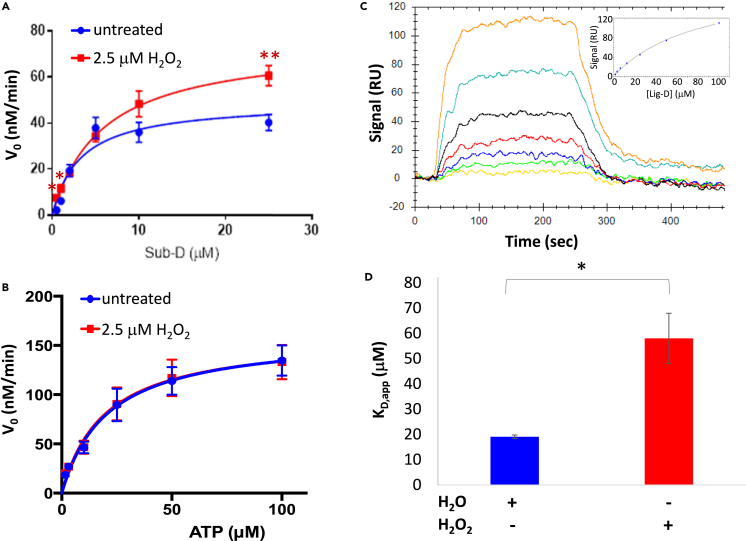
Figure 3.
Biochemical and Biophysical Analysis of ERK2-Sub-D Interactions
(A and B) Steady-state kinetics analysis of ERK2 toward Sub-D following pre-treatment of ERK2 with 2.5 μM H2O2 (red) or dH2O (untreated; blue). In (A), the concentration of Sub-D was varied while holding ATP constant at 20 μM while in (B), Sub-D was held constant at 1 μM while varying ATP levels. Error bars represent standard error about the mean (n > 3 independent experiments repeated in duplicate). Those points that exhibited a statistically significant difference in initial velocity (v0) between treated and untreated samples are indicated by an asterisk (∗; p < 0.05; ∗∗p < 0.01).
(C) Representative equilibrium binding experiment (from among 3 independent experiments done in duplicate) measuring the affinity of oxidized ERK2 for Lig-D using surface plasmon resonance (SPR) at Lig-D concentrations ranging from 1.6 to 100 μM. The binding curve is shown in the inset.
(D) Average KD,app for untreated ERK2 (blue) and ERK2 treated with 2.5 μM H2O2 before immobilization (n = 3 independent experiments repeated in duplicate). Error bars represent standard error about the mean. Statistically significant differences are indicated by an asterisk (∗, p < 0.05).