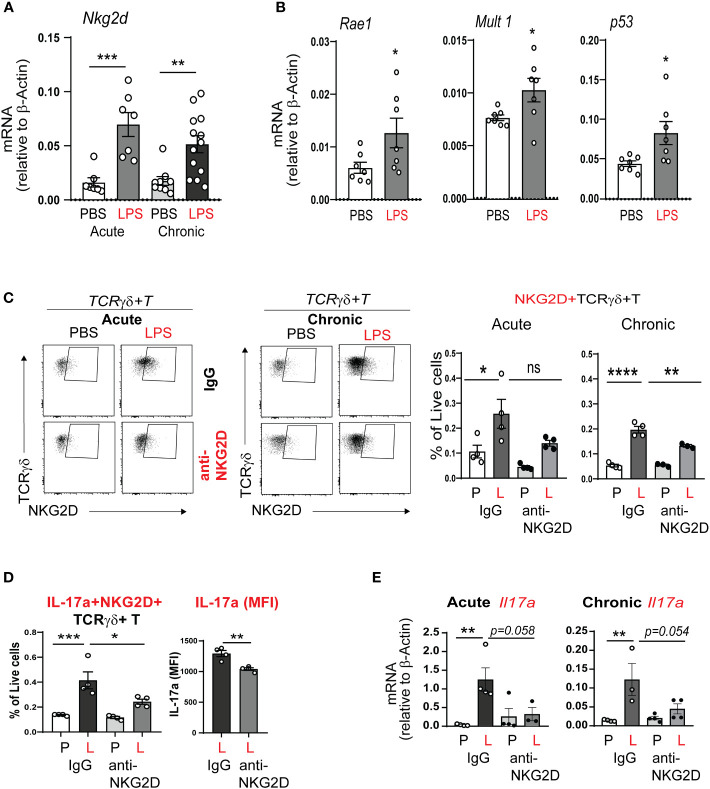
Figure 6.
NKG2D mediates acute and chronic neonatal LPS-induced γδ T cell activation and IL-17a production. (A) Nkg2d mRNA expression was increased in neonatal lung after acute and chronic exposure to LPS. (B) LPS induced neonatal lung mRNA expression of the NKG2D ligands Rae1 and Mult1, as well as the master regulator of DNA damage p53. (C, D) We inoculated immature C57BL/6J mice with LPS or PBS intranasally on DOL 3, 5, 7 and 10. One hour prior to each PBS or LPS treatment, mice were injected with IgG or anti-NKG2D antibody (Clone HMG2D) intraperitoneally. Lungs were examined by flow cytometry after acute and chronic LPS exposure. The NKG2D receptor expression, as a marker of activation, was detected on γδ T cells using a different clone of the anti-mouse NKG2D antibody (clone CX5). NKG2D+ γδ T cells were increased after acute and chronic LPS treatment in the neonatal lung, consistent with γδ T cell activation. IL-17a+NKG2D+ γδ T cells were also increased. Anti-NKG2D antibody treatment attenuated the effects of LPS on the activated NKG2D+ γδ T cells and the fraction of these cells that produced IL-17a. This analysis quantified native IL-17a expression only using a Protein Transportation Inhibitors cocktail. Per cell IL-17a expression (MFI) in NKG2D+ γδ T cells after LPS exposure was quantified and the effect of in vivo anti-NKG2D treatment was assessed. (E) Both acute and chronic LPS exposure induced whole lung Il17a mRNA expression and this effect was inhibited with in vivo anti-NKG2D treatment. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, ns – non-significant (one-way ANOVA and unpaired t test). Each open circle indicates one baby mouse. One of at least two independent experiments is presented.