Figure 1.
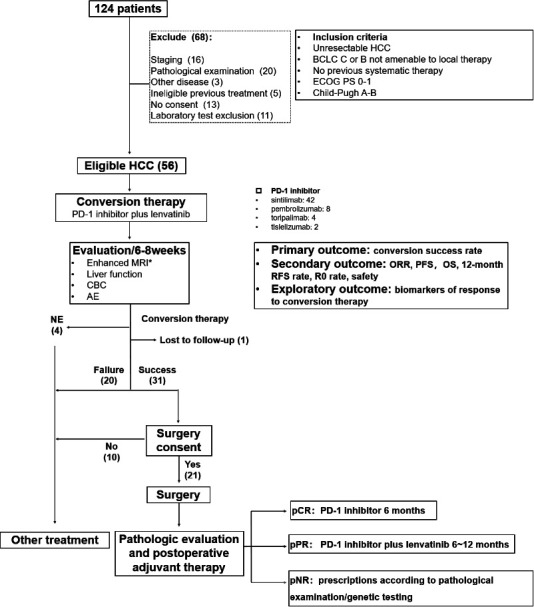
CONSORT (Consolidated Standards of Reporting Trials) diagram and study design. AE, adverse event; BCLC, Barcelona Clinic Liver Cancer; CBC, complete blood count; ECOG, Eastern Cooperative Oncology Group; HCC, hepatocellular carcinoma; NE, not estimable; ORR, objective response rate; OS, overall survival; pCR, pathological complete response (no viable tumor cells in the resection specimens, including completely resected primary tumors, tumor thrombosis, and lymph nodes); PD-1, programmed cell death protein-1; PFS, progression-free survival; PS, performance status; pNR, pathological non-response (> 50% viable tumor cells in the primary tumor or appearance of new lesions); pPR, pathological partial response (≤50% viable tumor cells in the primary tumor).
