Abstract
About a third of patients with inherited metabolic diseases with neurologic involvement suffer from a movement disorder, in the form of ataxia, hyperkinetic movements, or hypokinetic-rigid syndrome. We reviewed and updated the list of known metabolic etiologies associated with various types of movement disorders, and found approximately 200 relevant inborn errors of metabolism. This represents the first of a series of articles attempting to create and maintain a comprehensive list of clinical and metabolic differential diagnoses according to system involvement.
Keywords: Movement disorders, inborn errors of metabolism, inherited metabolic diseases, ataxia, dystonia, myoclonus, choreoathetosis, tremor, parkinsonism, hypokinetic-rigid syndrome
1. Introduction
It can be difficult to keep abreast of new developments in the field of inherited metabolic diseases. Given the rapid pace of advances in molecular diagnostics and a growing number of new diseases, we reason it is timely to provide a series of short reviews on inborn errors of metabolism (IEMs) involving different organs and systems. This series of articles will provide a rapidly accessible list of inherited metabolic diseases associated with specific signs and symptoms. The list will follow the classification as included in the knowledge base of IEMs (IEMBase) [1] and in the recently proposed Nosology of IEMs [2]. Just as the classic textbook “Gamuts in Radiology” and its subsequent updates have served as a comprehensive list of radiographic differential diagnosis for over four decades [3], we hope that our series of articles will serve as a comprehensive and updated list of metabolic differential diagnosis for the busy clinician.
The first issue of our series will be dedicated to movement disorders, which are not uncommon in patients with inherited metabolic diseases. In a prospective study of 170 patients with confirmed or highly suspected IEMs with neurologic involvement, 29% had a movement disorder [4]. This likely represents an underestimate, since many neurometabolic conditions now known to be associated with movement disorders had not yet been described by the time that article was published (particularly the group of disorders of complex lipid synthesis and remodeling, in which movement disorders are prevalent). A second reason why the aforementioned prevalence represents an underestimate is because many IEMs manifest with adult-onset movement disorders. Although the age of the patients evaluated in this series was not specified, it is likely that at least most of those patients were children, given that a pediatric department represented one of the sites where patients were evaluated.
A recent paper addressed the opposite question, i.e., what percentage of patients with a movement disorder has an IEM. Of a total of 378 patients with a movement disorder, ranging in age from birth to 84 years (mean: 31 years), 45 subjects had probable pathogenic variants in one of 71 genes included in the current nosology of IEMs. Another recent article evaluated the genetic landscape of movement disorders in 51 pediatric patients, 11 of whom were found to have an IEM [5]. This means that 9.3% of patients of any age with a movement disorder suffer from a metabolic etiology, while this percentage is as high as 22% in pediatric patients [6].
The burden of movement disorders caused by IEMs is significant and both clinical course and symptoms may overlap with non-metabolic diseases. The overall health-related quality of life (as evaluated by the Pediatric Quality of Life Inventory 4.0) was found to be significantly lower when compared to published data for children with chronic debilitating conditions, including malignancy, diabetes mellitus, rheumatic disease, and psychiatric disorders [7]. The overall adaptive functioning (as measured by the Vineland Adaptive Behavior Scale) was found to be 51.9%, thus suggesting a developmental age half that of the chronological age, indicating global impact of the IEM on brain function [7].
2. Signs and symptoms
Other than ataxia, movement disorders in children are typically divided in two subgroups: hyperkinetic/dyskinetic movement disorders (which include dystonia, chorea, athetosis, myoclonus, tremors, tics, and stereotypies), and hypokinetic movement disorders, which include Parkinsonism. A formal definition of each hyperkinetic movement disorder, and how to distinguish them from one another, is outside of the scope of the present article, and can be found elsewhere [8]. In the case of Parkinsonism, the preferred term in children is “hypokinetic-rigid syndrome”, as it’s often incomplete (resting tremor is very rare in pediatric Parkinsonism), complex (associated with other neurologic involvement), and the pathomechanism is not solely restricted to loss of presynaptic dopaminergic neurons [9,10].
In a case series of 50 patients with movement disorder due to inborn errors of metabolism, 54% of patients had dystonia, 28% had myoclonus, 14% had stereotypies, 10% each had chorea, athetosis, and tremor, and 4% had Parkinsonism [4]. In another series of 24 patients, 67% had dystonia, 29% suffered from myoclonus, and 25% had ataxia [7]. In this same series, 37.5% had a combination of two or more movement disorder, and the most common groups corresponded to organic acidurias (26%) and respiratory chain defects (21%) [7].
It is important to recognize that the character of the movement disorder can evolve over time. As an example, the dystonia seen in patients with glutaric aciduria type 1 evolves from mobile to fixed, and is ultimately associated with akinetic-rigid Parkinsonism [11].
The relative occurrence of specific movement symptoms in nine IEM categories is illustrated in Figure 1. The most common symptoms in 207 disorders were ataxia (73%), dystonia (47%), chorea/athetosis (24%), hypokinetic-rigid syndrome (17%), tremor (15%) and myoclonus (14%). Supplemental Table 1 summarizes in detail IEMs, including diagnostic markers, associated with movement disorders.
Figure 1.
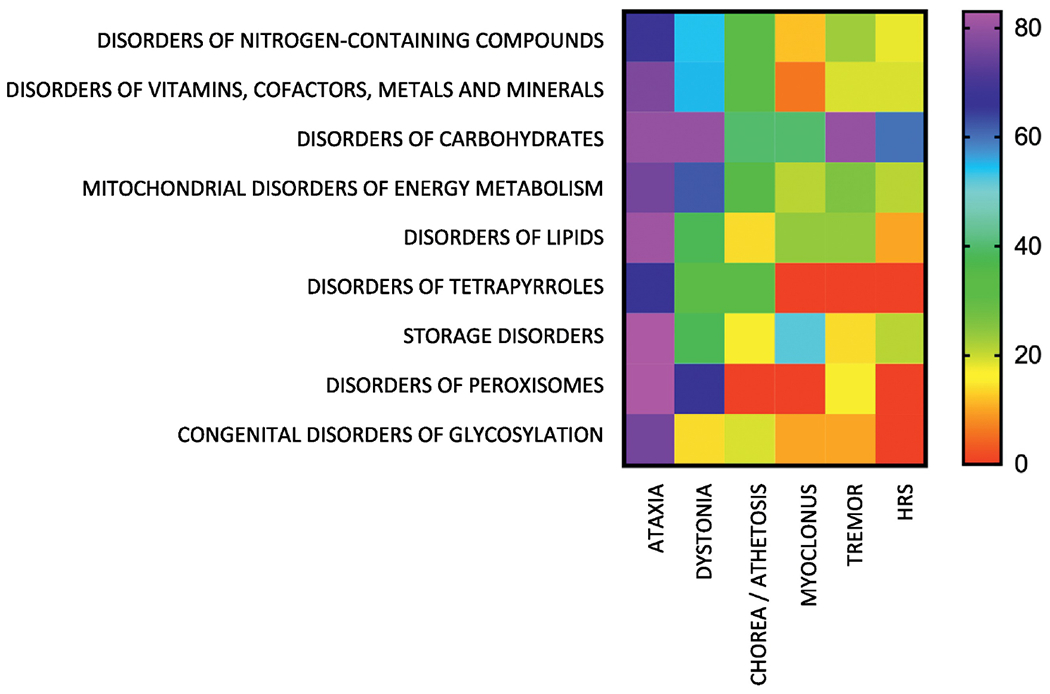
Occurrence (%) of symptoms associated with movement disorders in nine categories of IEMs. The percentages for each movement disorder were calculated using as the denominator the total number of IEMs in each category presenting with any movement disorder. Heat scale ranges from red (0%) for diseases with no particular symptoms reported to violet (100%) for diseases with particular symptoms being highly characteristic. HRS: hypokinetic-rigid syndrome.
3. Diagnosis/DD
An inherited metabolic disease should always be suspected when movement disorders remain unexplained, once more common etiologies such as drug side effects, infections, and focal brain lesions have been ruled out [9,12]. Red flags suggesting that a movement disorder might be caused by an IEM include: 1) early age of onset (the earlier the onset, the more likely a metabolic etiology); 2) associated neurologic or extraneurologic signs and symptoms; 3) progressive neurodegeneration; 4) consanguinity; 5) family history of similar disorder; 6) autonomic dysfunction; 7) paroxysmal episodic events; and 8) acute or subacute onset with or without triggering factors such as fasting, fever, or high-protein intake [12,13].
A list of laboratory investigations to aid in the diagnosis of the various listed IEMs is summarized in Table 1. For more details see Supplemental Table 1. The diagnostic approach, as with all IEMs, should focus on treatable disorders.
Table 1.
Biochemical investigations in movement disorders.
| Routine tests | Biochemical profiles | Special tests |
|---|---|---|
| Ammonia (B) | Amino acids (P, CSF) | Biogenic amines (CSF) |
| Blood count | Organic acids (U) | Pterins (CSF) |
| Glucose (P, CSF) | Purines and pyrimidines (U) | Folates (CSF) |
| Lactate (P, CSF) | Acylcarnitines (DBS, P) | Free thiamine (CSF) |
| Pyruvate (P) | B6-Vitamers | Lysosomal enzymes (DBS) |
| ASAT/ALAT (P) | VLCFA (P) | |
| CK (P) | Sialotransferins (S) | |
| ALP (P) | Vitamin E (P) | |
| Uric acid (U, P) | Sterols (P) | |
| Copper (S, U) |
4. Treatment
Therapy of movement disorders in patients with IEMs involves primary treatment of the underlying metabolic condition, as well as symptomatic treatment of the specific movement disorder, such as for example benzodiazepines, anticholinergic drugs, dopaminergic drugs, baclofen, botulinum toxin, or deep brain stimulation for dystonia.
Supplemental table 1 includes information on primary treatment options for the mentioned IEMs, i.e., treatment that addresses at least one aspect of the pathophysiology of the disease, when available.
5. Conclusion
Symptoms from neurometabolic diseases frequently overlap with those of other neurologic diseases. We provide a comprehensive list of neurometabolic diseases associated with movement disorders, as well as a proposed battery of standard laboratory and biochemical tests to aid in diagnosis based on the many possible IEMs in the aforementioned list.
We intend to write a limited series of concise educational summaries with up-to-date information on the differential diagnosis of IEMs. Our first entry in the series found a total of 207 inherited metabolic diseases associated with various types of movement disorders. The full list can be accessed for free at www.iembase.org/gamuts, and will be curated and updated on a regular basis. We hope that this tool will prove useful in the daily work of the metabolic clinician.
Supplementary Material
Supplemental table 1. Types of movement disorders described in inherited metabolic disorders. IEMs with movement disorder as a primary or prominent feature are in bold. For IEMs associated with multiple types of movement disorders, the most prevalent one is also marked in bold. Abbreviations: AASA, alpha-aminoadipic acid semialdehyde; ALP, alkaline phosphatase; B, blood; CSF, cerebrospinal fluid; ERT, enzyme replacement therapy; F, fibroblasts; HRS, hypokinetic-rigid syndrome; L, leukocytes; M, muscle; P, plasma; RBC, red blood cells; S, serum; U, urine.
* Oculogyric crises
** Orolingual/facial dyskinesia
*** Hyperekplexia
# Stereotyped fencing and/or bicycling movements
## Paroxysmal exercise-induced dyskinesia
### Midline hand movements
$ Myokimia
$$ Stereotypic/dyskinetic movements
$$$ Variously described as choreiform, athetoid, dystonic, myoclonic, or action tremor
Highlights.
We found 207 inherited metabolic diseases associated with various types of movement disorders, and provide a list of laboratory tests to consider to aid in the diagnosis of these diseases.
This represents the first issue in a series of concise educational summaries providing a comprehensive and updated list of metabolic differential diagnosis according to system involvement.
We will curate and update this list on a continual basis in the IEMbase website.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Lee JJY, Wasserman WW, Hoffmann GF, van Karnebeek CDM, Blau N, Knowledge base and mini-expert platform for the diagnosis of inborn errors of metabolism, Genet. Med. Off. J. Am. Coll. Med. Genet 20 (2018) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferreira CR, van Karnebeek CDM, Vockley J, Blau N, A proposed nosology of inborn errors of metabolism, Genet. Med. Off. J. Am. Coll. Med. Genet (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reeder MM, Bradley WGJ, Merritt CR, Reeder and Felson’s Gamuts in Radiology: Comprehensive Lists of Roentgen Differential Diagnosis, Springer; New York, 2003. https://books.google.com/books?id=NP9_9zxW73IC. [Google Scholar]
- [4].Gouider-Khouja N, Kraoua I, Benrhouma H, Fraj N, Rouissi A, Movement disorders in neuro-metabolic diseases, Eur. J. Paediatr. Neurol 14 (2010) 304–307. [DOI] [PubMed] [Google Scholar]
- [5].Cordeiro D, Bullivant G, Siriwardena K, Evans A, Kobayashi J, Cohn RD, Mercimek-Andrews S, Genetic landscape of pediatric movement disorders and management implications, Neurol. Genet 4 (2018) e265. doi: 10.1212/NXG.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Montaut S, Tranchant C, Drouot N, Rudolf G, Guissart C, Tarabeux J, Stemmelen T, Velt A, Fourrage C, Nitschké P, Gerard B, Mandel J-L, Koenig M, Chelly J, Anheim M, French Parkinson’s and Movement Disorders Consortium, Assessment of a Targeted Gene Panel for Identification of Genes Associated With Movement Disorders, JAMA Neurol. 75 (2018) 1234–1245. doi: 10.1001/jamaneurol.2018.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eggink H, Kuiper A, Peall KJ, Contarino MF, Bosch AM, Post B, Sival DA, Tijssen MAJ, de Koning TJ, Rare inborn errors of metabolism with movement disorders: a case study to evaluate the impact upon quality of life and adaptive functioning, Orphanet J. Rare Dis 9 (2014) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, Singer HS, Alter K, Ben-Pazi H, Butler EE, Chen R, Collins A, Dayanidhi S, Forssberg H, Fowler E, Gilbert DL, Gorman SL, Gormley ME, Jinnah HA, Kornblau B, Krosschell KJ, Lehman RK, MacKinnon C, Malanga CJ, Mesterman R, Michaels MB, Pearson TS, Rose J, Russman BS, Sternad D, Swoboda KJ, Valero-Cuevas F, Definition and classification of hyperkinetic movements in childhood, Mov. Disord. Off. J. Mov. Disord. Soc 25 (2010) 1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].García-Cazorla A, Ortez C, Pérez-Dueñas B, Serrano M, Pineda M, Campistol J, Fernández-Álvarez E, Hypokinetic-rigid syndrome in children and inborn errors of metabolism, Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc 15 (2011) 295–302. [DOI] [PubMed] [Google Scholar]
- [10].García-Cazorla A, Duarte ST, Parkinsonism and inborn errors of metabolism, J. Inherit. Metab. Dis 37 (2014)627–642. [DOI] [PubMed] [Google Scholar]
- [11].Gitiaux C, Roze E, Kinugawa K, Flamand-Rouvière C, Boddaert N, Apartis E, Valayannopoulos V, Touati G, Motte J, Devos D, Mention K, Dobbelaere D, Rodriguez D, Roubertie A, Chabrol B, Feillet F, Vidailhet M, Bahi-Buisson N, Spectrum of movement disorders associated with glutaric aciduria type 1: a study of 16 patients, Mov. Disord. Off. J. Mov. Disord. Soc 23 (2008) 2392–2397. [DOI] [PubMed] [Google Scholar]
- [12].Sedel F, Saudubray J-M, Roze E, Agid Y, Vidailhet M, Movement disorders and inborn errors of metabolism in adults: a diagnostic approach, J. Inherit. Metab. Dis 31 (2008) 308–318. [DOI] [PubMed] [Google Scholar]
- [13].Fernández-Álvarez E, Roubertie A, Movement disorders in childhood metabolic diseases, in: Jankovic J, Poewe W (Eds.), Mov. Disord. Neurol. Syst. Dis, Cambridge University Press, Cambridge, 2014: pp. 115–130. doi: 10.1017/CB09781139175845.010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1. Types of movement disorders described in inherited metabolic disorders. IEMs with movement disorder as a primary or prominent feature are in bold. For IEMs associated with multiple types of movement disorders, the most prevalent one is also marked in bold. Abbreviations: AASA, alpha-aminoadipic acid semialdehyde; ALP, alkaline phosphatase; B, blood; CSF, cerebrospinal fluid; ERT, enzyme replacement therapy; F, fibroblasts; HRS, hypokinetic-rigid syndrome; L, leukocytes; M, muscle; P, plasma; RBC, red blood cells; S, serum; U, urine.
* Oculogyric crises
** Orolingual/facial dyskinesia
*** Hyperekplexia
# Stereotyped fencing and/or bicycling movements
## Paroxysmal exercise-induced dyskinesia
### Midline hand movements
$ Myokimia
$$ Stereotypic/dyskinetic movements
$$$ Variously described as choreiform, athetoid, dystonic, myoclonic, or action tremor


