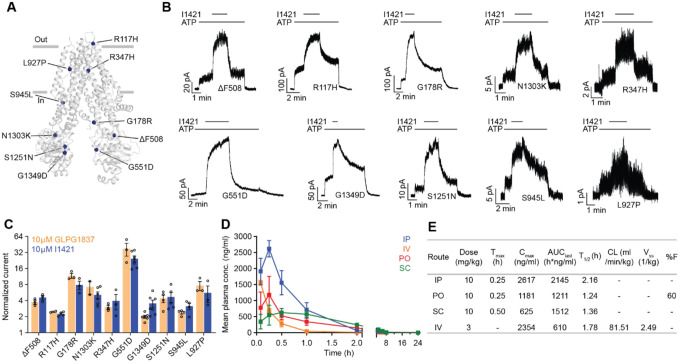
Figure 5: The activity of I1421 against 10 CF-causing mutations.
(a) The positions of the mutations mapped onto dephosphorylated and ATP-free CFTR (PDB 5UAK). (b) Representative macroscopic current traces in response to I1421 (10 μM) perfusion onto inside-out membrane patches excised from CHO cells. 3 mM ATP was used. (c) Potentiation activity of I1421 versus GLPG1837. The mean and SE values were determined from 2 to 7 patches. (d) Pharmacokinetic analysis of compound I1421. Plasma concentration-time profiles in male C57BL/6N mice following a single subcutaneous (SC), intraperitoneal (IP), per-oral (PO) (dose 10 mg/kg) or intravenous (IV) (3 mg/kg) administration. Data represent means and SDs. (e) Selected pharmacokinetic parameters of I1421. Cmax: peak plasma concentration; Tmax: the time when the peak plasma concentration was observed; AUClast: the areas under the concentration time curve; T1/2: terminal half-life; CL: clearance, Vss: steady-state volume of distribution; %F: %bioavailability.