Fig. 3: PEG/polyproline linkers (Promers) mitigate dye-dye interactions on peptides with multiple fluorophores.
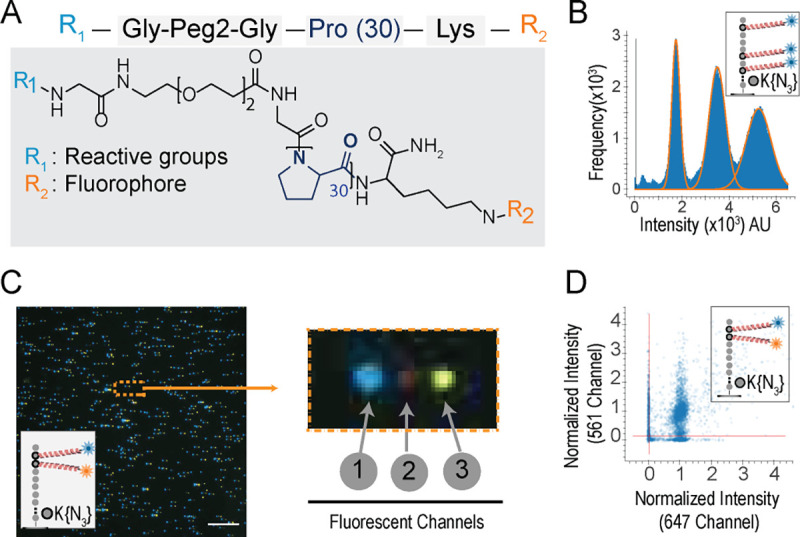
(A) illustrates Promer design and structure, with a 30-unit proline repeat flanked by a fluorophore (R2) linked to a lysine residue, and a DBCO reactive moiety (R1) linked via a flexible Glycine-PEG2-Glycine spacer. (B) The intensity histogram of 59,405 partially photobleached peptides (JSP126) shows three distinct peaks, indicating the resolution of one, two, or three active Atto643 fluorophores (installed via Promers at azido-lysine residues at amino acid positions 2, 6, and 8). The additive nature of the fluorescence intensities (median values for 1, 2, and 3 dyes, respectively, are 17,838; 35,040; and 52,242 arbitrary units) demonstrates minimal quenching between the fluorophores. (C) A representative TIRF micrograph (left panel) of individual JSP212 peptides, labeled with Atto643 (FRET acceptor) and JF549 (FRET donor) dyes on Promers at the 2nd and 3rd amino acid positions, demonstrates low FRET levels between the dyes. This composite image was made from offsetting the signal from three fluorescent channels (1-acceptor, 2-FRET, and 3-donor), shown enlarged for a single peptide molecule at right. The presence of signals in both donor and acceptor channels indicates the magnitude of FRET effect is less than 10% even on consecutive amino acids due to the Promers. (D) A scatter plot of fluorescence intensities across the donor and acceptor channels shows that 67% of individual peptide molecules (10,385 filtered peptides) exhibit two distinct fluorophores, confirming low FRET levels when dyes are tethered by Promers. Scale bar, 10 μm.
