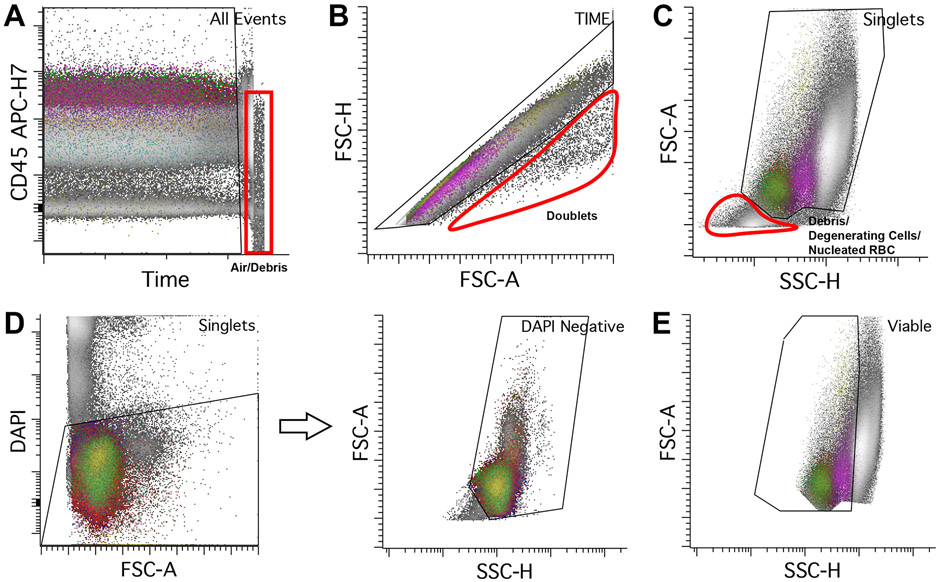
Figure 1.
Initial gating from all events to mononuclear cells. A. CD45 APC-H7 versus time. Stable acquisition has all populations showing consistent intensities of a single selected fluorochrome (in this case CD45 APC-H7) over time. Towards the end of the acquisition, there is an abrupt drop in CD45 intensity (red box) from acquisition of air or debris due to sample exhaustion. It is recommended to remove these events from downstream analysis. B. Doublets (circled in red) should be excluded from analysis. An individual single cell has defined area, height, and width. To gate for singlets, events should be plotted using FSC height versus area, using a roughly diagonal singlet gate as shown. Cell aggregates falling outside of this linear zone are aggregates which should be excluded from downstream analysis. C. Viable cells can be gated using FSC (linear scale) versus SSC (log scale) to exclude degenerated cells, nucleated red cells, and other debris (circled in red). D. DAPI can also be used to exclude non-viable cell material; all cells that are negative for DAPI are gated which should yield similar results compared to the method in C; we use DAPI as a viability gating tool in all samples from solid tissue. E. Gating of mononuclear cells using FSC (linear scale) versus SSC (log scale). Include lymphocytes (lowest FSC and SSC) and monocytes (intermediate FSC and SSC), and exclude granulocytes (highest FSC and SSC). Note that immature B-cells (hematogones) usually have lower FSC and SSC than mature lymphocytes. Other cells that are captured within the mononuclear gate include basophils, blasts, dendritic cells, and plasma cells.