Abstract
Background:
In 2015 the FDA approved transcarotid artery revascularization(TCAR) as an alternative to carotid endarterectomy(CEA) and transfemoral carotid artery stenting(TF-CAS) for high-risk patients with carotid stenosis. This was granted in the absence of level 1 evidence to support TCAR. We aimed to document trends in TCAR utilization, its diffusion over time, and the clinical phenotypes of patients undergoing TCAR, CEA, and TF-CAS.
Methods:
We used the Vascular Quality Initiative to study TCAR patients. We calculated the number of TCARs performed and the percent of TCAR utilization versus CEA/TF-CAS. Using data from before TCAR was approved, we calculated propensity scores for patients to receive CEA. We applied this model to patients undergoing carotid revascularization from 2016–2022 and grouped patients by the procedure they ultimately underwent, examining overlap in score distribution to measure patient similarity. We measured the trend of in-hospital stroke/death after TCAR.
Results:
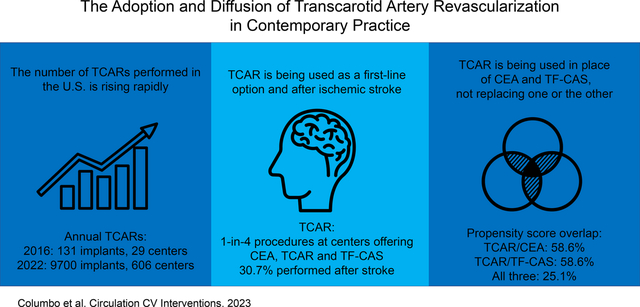
We studied 31,447 TCAR patients from 1/1/2016–3/31/2022. The number of centers performing TCAR increased from 29 to 606. In 2021, TCAR represented 22.5% of carotid revascularizations at centers offering all three procedures. The percentage of TCAR patients who met approved high-risk criteria decreased from 88.5% to 80.9%(p<0.001). Those with a prior ipsilateral carotid procedure decreased from 20.6% to 12.0%(p<0.001). Patients undergoing TCAR after stroke increased from 19.7% to 30.7%(p<0.001). Propensity-score overlap was 55.4% for TCAR/CEA, and 58.6% for TCAR/TF-CAS, demonstrating that TCAR patients have a clinical phenotype mixed between those who undergo CEA and TF-CAS. The average in-hospital stroke/death risk after TCAR was 2.3% in 2016 and 1.7% in 2022(p-trend:0.954).
Conclusions:
TCAR now represents nearly 1-in-4 procedures at centers offering it. TCAR was increasingly performed among standard-risk patients, and as a first-line procedural option after stroke. The absence of level 1 evidence underscores the importance of high-quality registry-based analyses to document TCAR’s real-world outcomes and durability.
Graphical Abstract:
Introduction:
Carotid artery stenosis remains a major risk factor for stroke, which is the fifth leading cause of death in the United States.1 Each year, approximately 100,000 carotid revascularization procedures are performed to reduce a patient’s future risk of stroke.2 Historically, the gold standard for carotid revascularization has been carotid endarterectomy (CEA), with transfemoral carotid artery stenting (TF-CAS) serving as a viable alternative for patients at average or low risk of complications with stenting.3–6
In 2015, transcarotid artery revascularization (TCAR) was approved by the FDA as a third procedural option among patients with carotid stenosis who were defined as high-risk.5, 7 The TCAR procedure involves a novel stenting technique, with a direct cervical carotid artery exposure and flow-reversal embolic protection.8, 9 Moreover, the TCAR procedure additionally obviates the need to traverse the aortic arch thereby eliminating any thromboembolic risks associated with this requisite maneuver during TF-CAS.7, 8 Observational studies of TCAR have shown promising short-term results in selected patients, with perioperative stroke or death rates similar to CEA and lower than that of TF-CAS.10–13
However, the optimal and current therapeutic role of TCAR in the contemporary management of patients with carotid stenosis remains undefined. TCAR was approved by the FDA via its 510(k) pathway, in the absence of high-quality level 1 evidence comparing TCAR’s safety and efficacy to CEA or TF-CAS.14 Whether patients now being selected for TCAR would have otherwise undergone CEA or TF-CAS is not known, blurring the distinction surrounding optimal procedure selection for stroke-risk reduction. Moreover, given the historically low rates of stroke after CEA, it is unclear whether a change in the carotid revascularization paradigm is even justified.4, 15 As such, the rightful place of TCAR in current practice remains a focus of controversy.
One prior study that examined the uptake of TCAR in the United States has important methodologic limitations, upon which our study seeks to partially overcome with more recent data.16 Accordingly, we aimed to define the change over time in the clinical profile of patients who underwent TCAR, which procedures it is being used in place of, and in doing so document the evolution of carotid interventions in contemporary practice.
Methods:
Human subjects protection
This study was approved by the Institutional Review Board at Dartmouth-Hitchcock Medical Center. All data were deidentified prior to analysis, and therefore the need for consent was waived.
Data source
We used data from the Vascular Quality Initiative (VQI) registry to study patients treated with TCAR. Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the VQI at www.vqi.org. The VQI collects granular real-world demographic, clinical, procedural, and outcome data from over 1000 centers in the United States and Canada on a variety of vascular procedures. As part of TCAR’s FDA approval process, the TCAR Surveillance Project was initiated, which stipulates that all patients undergoing TCAR have their procedural data entered into the VQI registry, with procedure reimbursement predicated on registry participation.17, 18 Accordingly, adjudication of billing information has demonstrated that >95% of all TCARs performed are captured by the registry.17 The Surveillance Project started in 2016, shortly following FDA approval of TCAR. Therefore, our study cohort reflects TCARs performed between January 1st 2016 and March 31st 2022.
Inclusion and exclusion criteria
We queried all patients in the registry who underwent TCAR. We then excluded patients who underwent TCAR for an indication other than carotid atherosclerotic or neointimal hyperplastic disease, such as stenting as part of a combined neurovascular procedure.
High-risk criteria definitions
The FDA-approved high-risk criteria for TCAR are based on the Centers for Medicare and Medicaid Services Decision Summary on carotid artery stenting, and include both clinical and anatomic characteristics.5 Clinical high-risk criteria are: NYHA class III or IV heart failure,5 left ventricular ejection fraction <30%,5 unstable angina or recent myocardial infarction,5 severe pulmonary disease with FEV1 <30%,19, 20 >80 years of age,19 or on hemodialysis.20 Anatomic high-risk criteria are: a contralateral carotid artery occlusion,5 history of prior ipsilateral CEA with restenosis,5 a contralateral laryngeal nerve palsy,20 prior neck irradiation,5 prior neck surgery,19, 20 or the presence of a tracheostomy.19, 20
Calculating TCAR utilization
We calculated the utilization of TCAR in two different but complementary ways. First, we determined the cumulative number of TCARs performed over time, and the cumulative number of centers performing them. We calculated this in quarterly intervals over the study period. This analysis included all patients who underwent TCAR for carotid atherosclerotic or neointimal hyperplastic disease, as noted above. Second, we calculated the percent of all carotid revascularization procedures performed (i.e., TCAR or CEA or TF-CAS) that were TCAR. The VQI captures information on CEA in one registry module, and information on TCAR and TF-CAS in another. Centers who perform TCAR are required to participate in the TCAR/TF-CAS module and report data on TCAR procedures. Conversely, centers may choose not to participate in the CEA module but still contribute data to the TF-CAS/TCAR module. Therefore, to avoid overrepresentation that may be present in a past study by other investigators,16 we calculated the relative percent of procedures that were TCAR among centers that participated in both registry modules. To allow centers to enter and exit the analysis (e.g., for a center that stopped participating in the VQI, or a center that newly enrolled), we calculated these percentages at quarterly intervals over the study period.
We also sought to determine whether proceduralists who were more familiar with carotid stenting prior to TCARs introduction were more likely adopt TCAR. To do this we limited the cohort to proceduralists with data in 2015 (prior to the inception of TCAR) and in 2020. We calculated the percent of procedures performed at the proceduralist-level in 2015 that were TF-CAS. We then calculated the percent of procedures that those individuals performed in 2020 that were TF-CAS, TCAR, or CEA, respectively, to determine whether TF-CAS use was associated with future TCAR use.
Propensity-score overlap
We used propensity-score overlap as a measure of similarity to understand the clinical phenotypes of patients selected to undergo TCAR, and which procedure they would have been most likely to undergo in the absence of TCAR (i.e., CEA or TF-CAS).21 To calculate the propensity scores we created a logistic regression model using data from 2015 (i.e., prior to the TCAR Surveillance Project) where the outcome was the procedure type (i.e., CEA or TF-CAS).22 The propensity score model included the covariates of age, gender, body mass index, the presence of focal neurologic symptoms, coronary artery disease, congestive heart failure, prior cardiac revascularization, hypertension, chronic obstructive pulmonary disease, home oxygen use, diabetes, chronic kidney disease, smoking status, prior ipsilateral carotid procedure, prior contralateral carotid procedure, preoperative aspirin, p2y12 inhibitor, dual antiplatelet, statin, beta blocker, anticoagulant, or ACE inhibitor use, preoperative functional status, anatomic high risk, elective versus urgent procedure, ASA class, general versus local anesthesia, intraoperative heparin, and intraoperative protamine use.
We then applied this model to the study period data from 2016 to 2022 and calculated the propensity scores for all patients. The C-statistic for these models remained high through all years of data, ranging from 0.94–0.99. We then grouped patients by the actual observed procedure that they underwent (i.e., TCAR, CEA, or TF-CAS) and calculated the overlap in propensity score probability distribution functions between the groups. The score overlap (i.e., common area) is a similarity measure between two or more probability density functions which allows measurement of the similarity between two or more populations.23 The score overlap ranges from 0% to 100%. An overlap of 0%, or no overlap, can be interpreted as 0% similarity between the groups based on the propensity score model. An overlap of 100%, or complete overlap, can be interpreted as 100% similarity in procedure-receipt propensity between the groups. This technique allows us to indirectly infer whether patients who underwent TCAR are similar in clinical profile to those who underwent CEA, TF-CAS, neither, or both and, by extension, the likely procedure that patients would have undergone had TCAR not been available.24–26
Statistical analysis
We described continuous variables with means and standard deviations (SD) and compared patients across years with one-way ANOVA testing. We reported categorical variables as percentages and compared patients across years with Chi-squared analysis. We used kernel density estimators to approximate the probability distribution functions for the fitted propensity scores. We used a normal distribution quantile transformation to visually display the distribution of the scores, which allows for enhanced visualization of the score overlap proportion (of the extent to which patients who received one procedure resemble those who received another procedure).21, 23 We performed analyses with the overall cohort, and after stratifying the cohort into high-risk and standard-risk groups to examine if the diffusion of patients to TCAR occurred first among high-risk patients (TCAR’s FDA-approved population) and then spilled-over to standard-risk patients. We calculated the unadjusted in-hospital risk of stroke or death over time as a moving average of the risk for that quarter and the previous three quarters. For example, the number reported in Q2 2017 is the average of Q3, Q4, 2016, and Q1, Q2 2017. We additionally calculated the annual rate of in-hospital stroke or death after TCAR as a function of volume at the proceduralist and center-level. To do this we grouped proceduralists and centers by their respective annual TCAR volume (e.g., <11, 11–20, or >20) and calculated the risk of perioperative stroke or death after TCAR. Missing data was less than 2% for all variables. We used R version 4.1.2 for statistical analysis (The R Foundation for Statistical Computing).
Results:
Patient characteristics and changes over time
We studied 31,447 patients who underwent TCAR from January 2016 to March 2021 (Table 1). All TCAR patients underwent their procedure in the United States. Across all patients, the mean age was 73.4 ± 9.0 years, 36.7% were female, and 90.1% were White. TCAR was performed for symptomatic patients with carotid stenosis in 46.5% of cases. Most procedures were performed on an elective basis (88.4%) and under general anesthesia (83.9%). Half of patients were active smokers (50.4%). Comorbidities were common among the cohort and as expected for this patient population. Characteristics of patients who underwent CEA and TF-CAS over the same period are described in Table S1 and S2 respectively.
Table 1:
Characteristics of patients who underwent TCAR by year, and overall.
| Variable | Year | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 [Q1] | Totals | ||||||||||
| n=131 | % | n=1377 | % | n=3579 | % | n=6619 | % | n=7709 | % | n=9700 | % | n=2332 | % | p-value | n=31,447 | % | |
|
|
|||||||||||||||||
| Age mean (SD), years | 72.7 | 9.5 | 72.7 | 9.7 | 73.4 | 8.8 | 73.2 | 9.0 | 73.2 | 9.0 | 73.6 | 9.0 | 73.8 | 8.8 | <0.001 | 73.4 | 9.0 |
| Female | 41 | 31.3 | 497 | 36.1 | 1318 | 36.8 | 2412 | 36.4 | 2760 | 35.8 | 3653 | 37.7 | 867 | 37.2 | 0.184 | 11548 | 36.7 |
| Race | |||||||||||||||||
| White | 124 | 95.4 | 1259 | 91.5 | 3219 | 90.0 | 5996 | 90.7 | 6913 | 89.7 | 8714 | 89.9 | 2101 | 90.2 | 0.089 | 28326 | 90.1 |
| Black or African American | 4 | 3.1 | 56 | 4.1 | 173 | 4.8 | 286 | 4.3 | 413 | 5.4 | 531 | 5.5 | 114 | 4.9 | <0.001 | 1577 | 5.0 |
| Unknown/Other | 1 | 0.8 | 33 | 2.4 | 127 | 3.6 | 230 | 3.5 | 266 | 3.5 | 267 | 2.8 | 60 | 2.6 | 0.004 | 984 | 3.1 |
| Asian | 1 | 0.8 | 11 | 0.8 | 28 | 0.8 | 61 | 0.9 | 76 | 1.0 | 100 | 1.0 | 31 | 1.3 | 0.493 | 308 | 1.0 |
| American Indian or Alaskan Native | 0 | 0.0 | 14 | 1.0 | 17 | 0.5 | 23 | 0.3 | 16 | 0.2 | 42 | 0.4 | 10 | 0.4 | <0.001 | 122 | 0.4 |
| More than 1 race | 0 | 0.0 | 3 | 0.2 | 9 | 0.3 | 8 | 0.1 | 9 | 0.1 | 27 | 0.3 | 9 | 0.4 | 0.061 | 65 | 0.2 |
| Native Hawaiian or Pacific Islander | 0 | 0.0 | 0 | 0.0 | 4 | 0.1 | 8 | 0.1 | 10 | 0.1 | 8 | 0.1 | 5 | 0.2 | 0.554 | 35 | 0.1 |
| Symptomatic* | 47 | 38.2 | 587 | 43.4 | 1596 | 45.5 | 2895 | 44.9 | 3534 | 46.9 | 4501 | 47.5 | 1113 | 48.5 | <0.001 | 14273 | 46.5 |
| Stroke | 25 | 19.7 | 347 | 25.4 | 968 | 27.3 | 1849 | 28.3 | 2278 | 29.9 | 2948 | 30.7 | 750 | 32.4 | <0.001 | 9165 | 29.5 |
| Cortical transient ischemic attack | 15 | 12.1 | 222 | 16.4 | 562 | 16.0 | 952 | 14.6 | 1197 | 15.8 | 1406 | 14.7 | 347 | 15.1 | 0.165 | 4701 | 15.2 |
| Amaurosis | 11 | 8.9 | 88 | 6.5 | 211 | 6.0 | 350 | 5.4 | 444 | 5.9 | 560 | 5.9 | 117 | 5.1 | 0.242 | 1781 | 5.8 |
| Vertebrobasilar or other | 6 | 4.7 | 36 | 2.6 | 83 | 2.3 | 153 | 2.3 | 166 | 2.2 | 227 | 2.4 | 65 | 2.8 | 0.353 | 736 | 2.4 |
| Prior ipsilateral carotid procedure | 27 | 20.6 | 256 | 18.6 | 565 | 15.8 | 908 | 13.7 | 1091 | 14.2 | 1167 | 12.0 | 258 | 11.1 | <0.001 | 4272 | 13.6 |
| Prior contralateral carotid procedure | 15 | 11.5 | 218 | 15.8 | 551 | 15.4 | 931 | 14.1 | 1072 | 13.9 | 1299 | 13.4 | 282 | 12.1 | 0.002 | 4368 | 13.9 |
| Anatomic high risk | 61 | 46.9 | 573 | 41.8 | 1566 | 44.1 | 3093 | 47.0 | 3539 | 46.1 | 4205 | 43.5 | 945 | 40.6 | <0.001 | 13626 | 43.5 |
| Medical high risk | 69 | 53.1 | 797 | 58.2 | 1989 | 55.9 | 3488 | 53.0 | 4134 | 53.9 | 5454 | 56.5 | 1250 | 53.7 | <0.001 | 17181 | 54.9 |
| Both | 15 | 11.5 | 273 | 19.9 | 675 | 19.0 | 1110 | 16.9 | 1223 | 15.9 | 1618 | 16.8 | 386 | 16.6 | <0.001 | 5300 | 16.9 |
| Standard risk † | 15 | 11.5 | 144 | 10.5 | 504 | 14.2 | 1256 | 19.1 | 1504 | 19.6 | 1847 | 19.1 | 517 | 22.2 | <0.001 | 5787 | 18.5 |
| Coronary artery disease | 62 | 47.7 | 696 | 50.5 | 1825 | 51.0 | 3382 | 51.2 | 3988 | 51.8 | 5083 | 52.4 | 1160 | 49.7 | 0.220 | 16196 | 51.5 |
| Congestive heart failure | 26 | 19.8 | 258 | 18.7 | 633 | 17.7 | 1089 | 16.5 | 1262 | 16.4 | 1642 | 16.9 | 368 | 15.8 | 0.128 | 5278 | 16.8 |
| Prior coronary revascularization | 50 | 38.2 | 582 | 42.3 | 1431 | 40.0 | 2602 | 39.3 | 3042 | 39.5 | 3808 | 39.3 | 853 | 36.6 | 0.040 | 12368 | 39.3 |
| Hypertension | 115 | 87.8 | 1248 | 90.6 | 3216 | 90.0 | 6042 | 91.4 | 6989 | 90.7 | 8942 | 92.2 | 2164 | 92.8 | <0.001 | 28716 | 91.4 |
| Chronic obstructive pulmonary disease | 29 | 22.3 | 375 | 27.2 | 989 | 27.6 | 1725 | 26.1 | 1894 | 24.6 | 2398 | 24.7 | 531 | 22.8 | <0.001 | 7941 | 25.3 |
| Oxygen | 3 | 2.3 | 49 | 3.6 | 135 | 3.8 | 212 | 3.2 | 246 | 3.2 | 331 | 3.4 | 67 | 2.9 | 0.505 | 1043 | 3.3 |
| Diabetes | 40 | 30.5 | 510 | 37.0 | 1358 | 38.0 | 2542 | 38.4 | 3015 | 39.1 | 3713 | 38.3 | 893 | 38.3 | 0.368 | 12071 | 38.4 |
| Chronic kidney disease | 11 | 8.4 | 93 | 6.8 | 211 | 5.9 | 394 | 6.0 | 509 | 6.6 | 594 | 6.1 | 125 | 5.4 | 0.230 | 1937 | 6.2 |
| Smoking | |||||||||||||||||
| Never | 33 | 25.4 | 321 | 23.3 | 970 | 27.1 | 1738 | 26.3 | 2099 | 27.2 | 2834 | 29.2 | 747 | 32.1 | <0.001 | 8742 | 27.8 |
| Prior | 34 | 26.2 | 346 | 25.2 | 774 | 21.6 | 1438 | 21.7 | 1673 | 21.7 | 2088 | 21.5 | 465 | 20.0 | 0.017 | 6818 | 21.7 |
| Current | 63 | 48.5 | 708 | 51.5 | 1833 | 51.2 | 3436 | 52.0 | 3935 | 51.1 | 4772 | 49.2 | 1117 | 48.0 | 0.002 | 15864 | 50.5 |
| Obese | 45 | 37.8 | 452 | 33.3 | 1201 | 33.6 | 2193 | 33.2 | 2548 | 33.1 | 3221 | 33.2 | 762 | 32.7 | 0.942 | 10422 | 33.2 |
| Preop medications | |||||||||||||||||
| Aspirin | 113 | 86.3 | 1241 | 90.1 | 3192 | 89.2 | 5946 | 89.8 | 6929 | 89.9 | 8747 | 90.2 | 2073 | 88.9 | 0.315 | 28241 | 89.8 |
| P2Y12 inhibitor | 114 | 87.7 | 1168 | 84.8 | 3072 | 85.9 | 5815 | 87.9 | 6792 | 88.1 | 8689 | 89.6 | 2093 | 89.8 | <0.001 | 27743 | 88.2 |
| DAPT | 102 | 77.9 | 1078 | 78.3 | 2799 | 78.3 | 5353 | 80.9 | 6226 | 80.8 | 7992 | 82.4 | 1910 | 81.9 | <0.001 | 25460 | 81.0 |
| Statin | 120 | 91.6 | 1215 | 88.2 | 3170 | 88.6 | 5978 | 90.4 | 6927 | 89.9 | 8746 | 90.2 | 2130 | 91.3 | 0.004 | 28286 | 90.0 |
| Beta-blocker | 71 | 54.6 | 772 | 56.1 | 2050 | 57.3 | 3771 | 57.1 | 4281 | 55.6 | 5481 | 56.5 | 1289 | 55.3 | 0.443 | 17715 | 56.4 |
| Anticoagulation | 12 | 9.2 | 198 | 14.4 | 519 | 14.5 | 939 | 14.2 | 1138 | 14.8 | 1467 | 15.1 | 385 | 16.5 | 0.067 | 4658 | 14.8 |
| ACE inhibitor | 63 | 48.1 | 737 | 53.6 | 1912 | 53.4 | 3485 | 52.7 | 4068 | 52.8 | 5143 | 53.0 | 1318 | 56.5 | 0.040 | 16726 | 53.2 |
| Preop functional status | |||||||||||||||||
| Independent | 106 | 93.8 | 1295 | 96.3 | 3383 | 96.4 | 6272 | 96.4 | 7304 | 95.9 | 9238 | 95.7 | 2215 | 95.9 | 0.162 | 29813 | 96.0 |
| Assist required | 6 | 5.3 | 49 | 3.6 | 124 | 3.5 | 222 | 3.4 | 308 | 4.0 | 396 | 4.1 | 91 | 3.9 | 0.271 | 1196 | 3.9 |
| Unable to get out of bed | 1 | 0.9 | 1 | 0.1 | 3 | 0.1 | 9 | 0.1 | 6 | 0.1 | 20 | 0.2 | 4 | 0.2 | 0.093 | 44 | 0.1 |
| Urgency | |||||||||||||||||
| Elective | 122 | 93.1 | 1260 | 91.6 | 3205 | 89.6 | 5951 | 89.9 | 6727 | 87.3 | 8477 | 87.4 | 2059 | 88.3 | <0.001 | 27801 | 88.4 |
| Urgent | 9 | 6.9 | 112 | 8.1 | 365 | 10.2 | 649 | 9.8 | 964 | 12.5 | 1196 | 12.3 | 265 | 11.4 | <0.001 | 3560 | 11.3 |
| Emergent | 0 | 0.0 | 4 | 0.3 | 9 | 0.3 | 16 | 0.2 | 17 | 0.2 | 26 | 0.3 | 8 | 0.3 | 0.953 | 80 | 0.3 |
| General Anesthesia | 93 | 71.5 | 1079 | 78.5 | 2832 | 79.2 | 5431 | 82.1 | 6528 | 84.7 | 8339 | 86.0 | 2078 | 89.1 | <0.001 | 26380 | 83.9 |
| Intraoperative heparin | 127 | 98.4 | 1348 | 98.6 | 3526 | 98.7 | 6540 | 99.0 | 7650 | 99.4 | 9649 | 99.5 | 2319 | 99.5 | <0.001 | 31159 | 99.2 |
| Intraoperative protamine | 86 | 71.7 | 1005 | 75.6 | 2708 | 77.5 | 5475 | 84.2 | 6759 | 89.0 | 8670 | 90.5 | 2108 | 91.5 | <0.001 | 26811 | 86.7 |
Patients may have more than one symptomatic subtype upon presentation.
Patients with neither anatomic or medical high risk criteria. SD: Standard deviation; Anatomic / Medical high risk as defined by the Centers for Medicare and Medicaid Services
The distribution of several important characteristics evolved over the study period (Table 1). The percent of patients who underwent TCAR in the setting of stroke increased from 19.7% in 2016 to 30.7% in 2021 (p<0.001). The percent of standard-risk patients undergoing TCAR (e.g., no anatomic or medical high-risk criteria) increased from 11.5% in 2016 to 19.1% in 2021 (p<0.001). While the absolute number of patients who underwent TCAR that had a history of a prior ipsilateral carotid procedure increased from n=27 in 2016 to n=1167 in 2021 (p<0.001), the relative percent of these patients to the total number of TCARs decreased from 20.6% to 12.0% over the same time interval (p<0.001).
TCAR utilization
The number of centers performing TCAR increased dramatically over the study interval, from 29 centers at the end of 2016, to 606 centers in March 2022 (Figure 1, Figure S1). The total number of implants similarly increased, from 131 at the end of 2016, to a total of 31,447 at the end of March 2022. TCAR utilization was most common in the Eastern U.S. from 2016–2018, then becoming most common in the Southern U.S. from 2019 onward, suggesting that uptake diffused geographically (Table S3, Figure S2).
Figure 1:
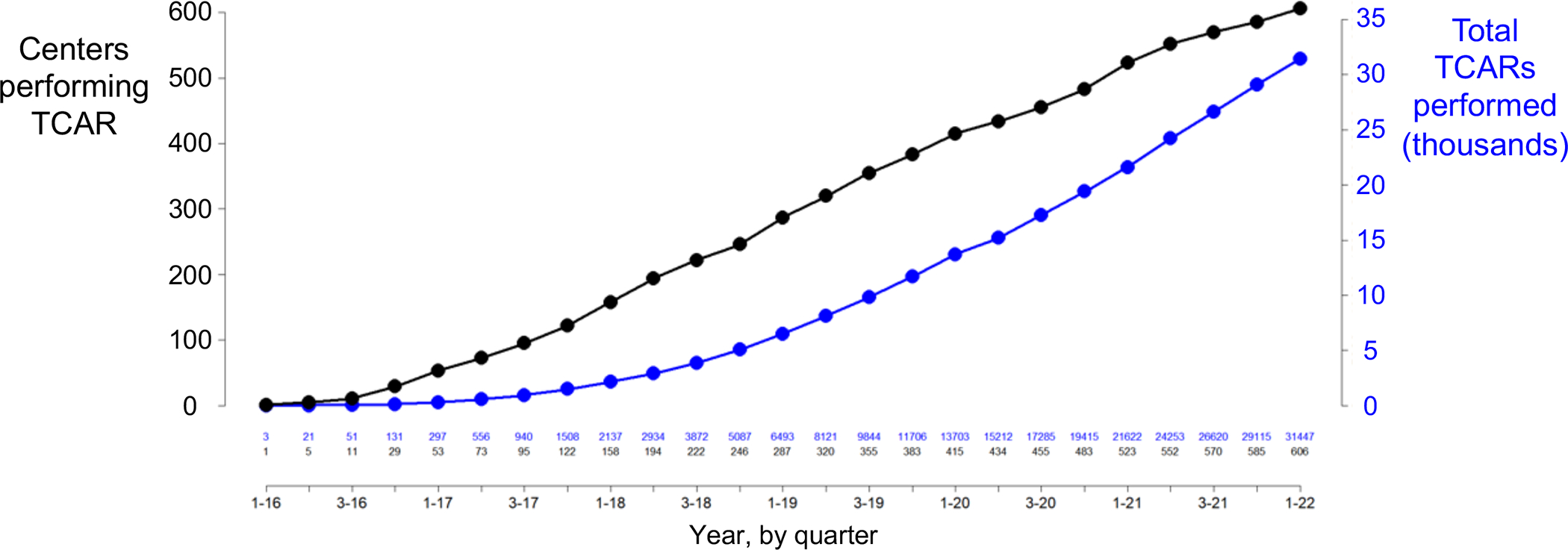
Number of transcarotid artery revascularizations (TCAR)s implanted and the number of centers performing them over time.
We performed a subanalysis stratifying the cohort into high-risk and standard-risk subgroups. TCAR use was most common among high-risk patients and increased from 115 implants across 27 centers at the end of 2016, to 25,507 implants at 592 centers at the end of March 2022 (Figure S3). TCAR was less commonly used among standard-risk patients, but still had 5,787 implants across 480 centers by the end of March 2022 (Figure S4).
We then restricted the cohort to centers reporting TCAR, CEA, and TF-CAS to estimate the percent market share of TCAR out of the total carotid revascularization procedures being performed (Figure 2). This revealed that TCAR accounted for an incrementally growing percentage of the total carotid interventions. In quarter 1 of 2016, TCAR represented 1.0% of all carotid procedures. This increased steadily over time to 23.9% in quarter 1 of 2022 (p-trend <0.001), and this curve did not appear to flatten. Stratifying this analysis into high and standard-risk subgroups revealed that TCAR represented 42.1% of procedures performed in high-risk patients by the end of March 2022, becoming more common than CEA in quarter 2 2020 (Figure S5). TCAR represented 10.8% of carotid revascularization procedures performed on standard-risk patients by the end of the study interval (Figure S6). TCAR use was similar across variable levels of TF-CAS use (Table S4).
Figure 2:
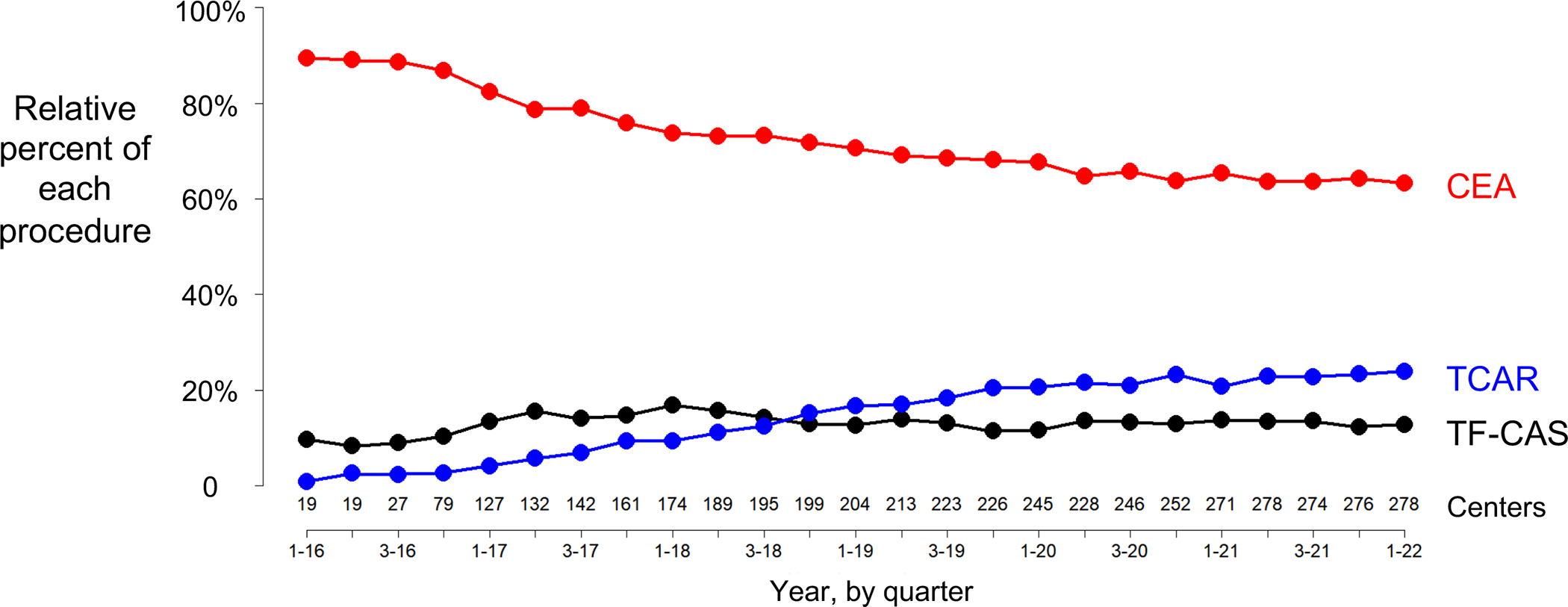
Relative percent market share of transcarotid artery revascularization (TCAR), carotid endarterectomy (CEA), and transfemoral carotid artery stenting (TF-CAS) among centers reporting all procedures.
We examined whether proceduralists who were using TF-CAS in 2015 prior to TCARs introduction were more likely to be future users of TCAR in 2020. We did not find a significant correlation between 2015 TF-CAS use and those proceduralists 2020 TCAR use (p=0.841) (Figure S7).
Propensity-score overlap between procedure groups
We calculated propensity score overlap between patients who underwent TCAR, CEA, and TF-CAS. This technique visually displays the distribution in the propensity scores and allows us to infer whether patients who underwent TCAR have a similar clinical profile to those who underwent CEA, TF-CAS, neither, or both and, by extension, the likely procedure that TCAR patients would have undergone had TCAR not been available (Figure 3).21, 23 The score overlap shared by TCAR, CEA, and TF-CAS was 25.1% (Figure 3, Region A). The score overlap was 58.6% for TCAR and TF-CAS (Figure 3, Region B), and for TCAR and CEA was 55.4% (Figure 3, Region C). These findings demonstrate that patients who are selected for TCAR are similar in clinical profile to patients who undergo TF-CAS, and to patients who undergo CEA, indicating that TCAR is being used in place of both procedures, rather than replacing one or the other. We performed a sensitivity analysis including region as a random effect and obtained similar results.
Figure 3:
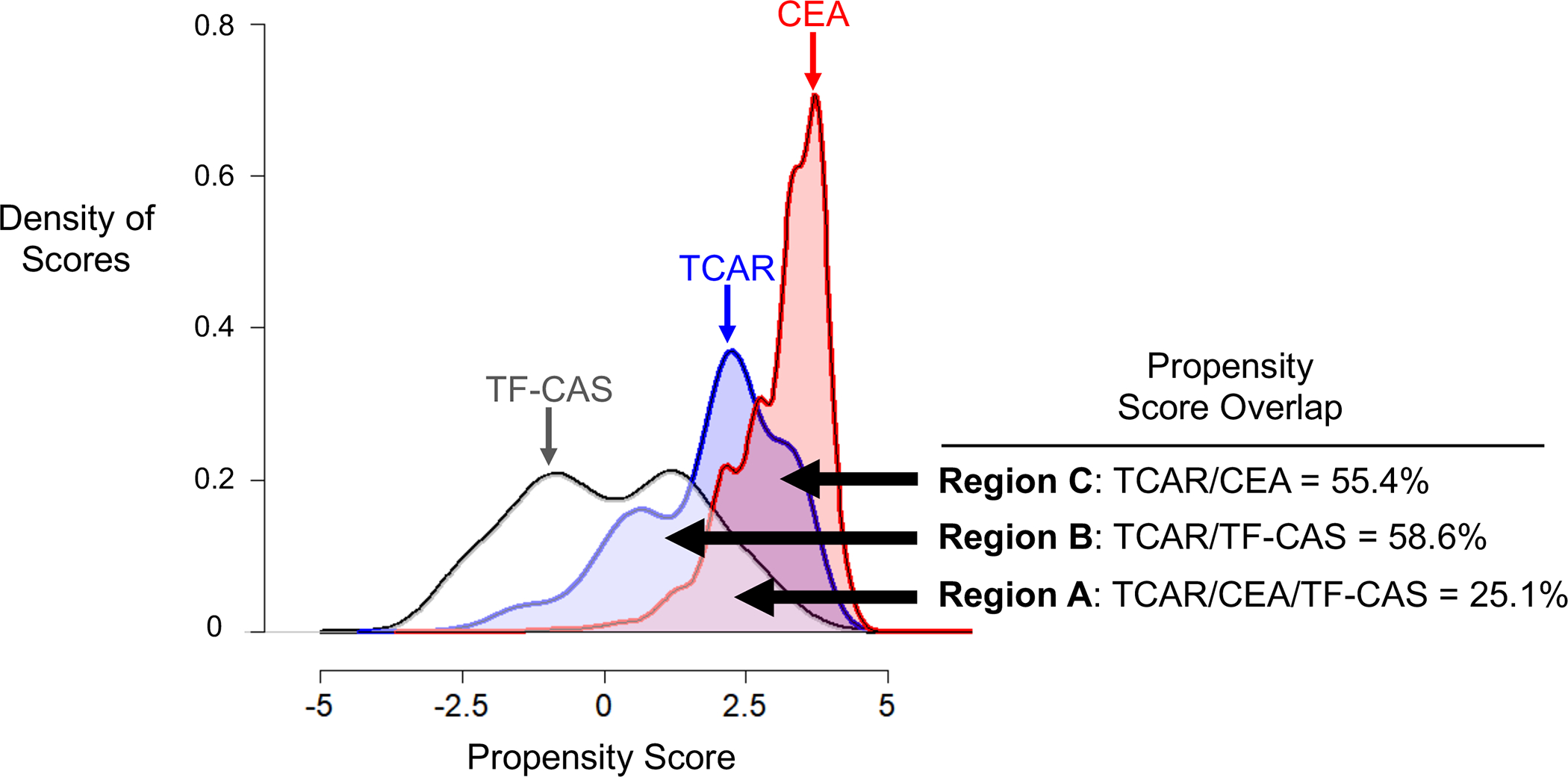
Density of propensity scores in a logarithmic scale of patients receiving transcarotid artery revascularization (TCAR), carotid endarterectomy (CEA), and transfemoral carotid artery stenting (TF-CAS), demonstrating that patients receiving TCAR are similar to either CEA or TF-CAS patients, while patients receiving CEA and TF-CAS are relatively distinct from each other.
We performed a subanalysis stratified by high- versus standard-risk patients. Among high-risk patients, the score overlap common to TCAR, CEA, and TF-CAS was 24.8%, for TCAR and TF-CAS was 59.0%, and for TCAR and CEA was 54.0% (Figure S8). Among standard-risk patients, the overlap common to all three procedures was 33.0%, for TCAR and TF-CAS was 51.0%, and for TCAR and CEA was 70.0% (Figure S9). These stratified results reveal that there is less overlap among high-risk patients, indicating that these patients are more definitively sorting into distinct procedures. Conversely, there is greater overlap among standard-risk patients, indicating that procedure choice here is more discretionary.
Trends in stroke or death
We examined the in-hospital perioperative risk of stroke or death over time. We calculated the moving average by quarter from 2016 through March 2022. The moving average of in-hospital risk of stroke or death after TCAR was 2.3% in quarter 4 2016 and 1.7% in quarter 1 2022 (p-trend: 0.954; Figure 4). We did not find a statistically significant association between annual center-level TCAR volume and the risk of in-hospital stroke/death after TCAR. There was a small but statistically significant association between annual proceduralist-level TCAR volume and the risk of in-hospital stroke or death (<11 TCARs: 1.89%, 11–20 TCARs: 1.47%, >20 TCARs: 1.47%; p<0.001) (Table S5).
Figure 4:
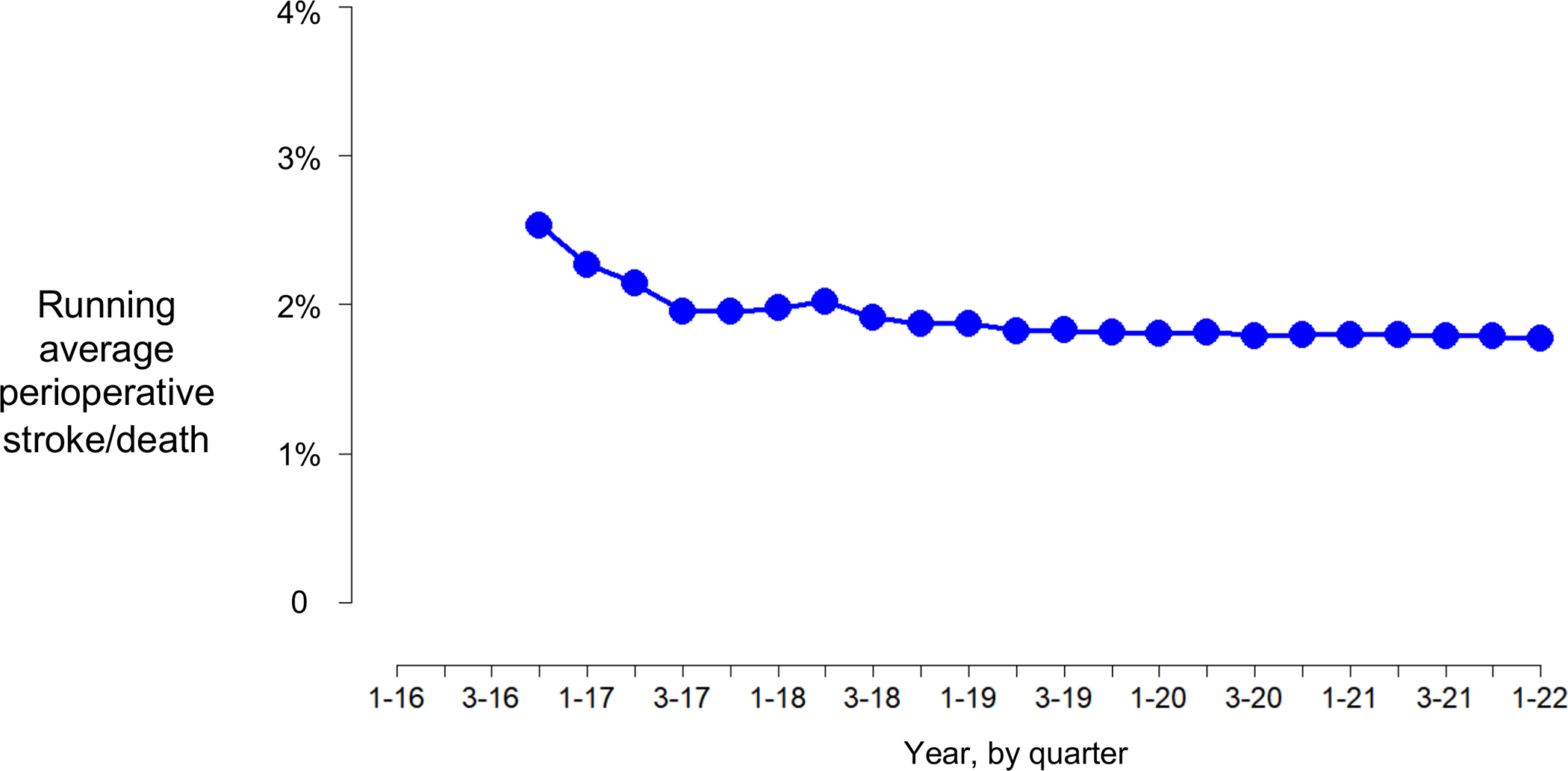
Moving average risk of in-hospital stroke or death after transcarotid artery revascularization (TCAR) over time.
Note: Due to the low number of events, each point estimate represents the average of that quarter and the previous three quarters (e.g., the point estimate for quarter 3, 2017, is the average of quarter 4 2016, and quarters 1–3 2017).
Discussion:
We found that TCAR had been performed at 606 centers by March 2022, with over 31,000 patients having undergone the procedure. Moreover, we identified a more than 74-fold increase in annual implants from 2016 to 2021, with TCAR now constituting nearly 1-in-4 carotid revascularizations at centers performing all three procedures. These findings indicate that TCAR has now assumed a major role in the contemporary management of carotid artery stenosis in the United States.
The observed explosive growth of TCAR across the United States appears to partially reflect a more liberal application of its use over time. TCAR was initially approved by the FDA in 2015 as a procedural alternative to CEA and TF-CAS for patients with high-risk anatomic or medical conditions.7, 27 However, we interestingly noted that an increasing number of patients who underwent TCAR had no documented high-risk criteria, accounting for more than 5,700 implants across 480 centers over the study interval. This appears to reflect spillover diffusion from the initially targeted TCAR population. Despite this, we found that the risk of in-hospital stroke and death after TCAR remains less than 2% across proceduralist and center-level volume strata. This low rate of adverse events has likely contributed to TCARs rapid adoption. Moreover, TCAR was increasingly used as a first-line treatment option, as patients with a history of a prior ipsilateral carotid procedure decreased by 40% over the study interval. This finding suggests that the rapid uptake in TCAR is at least in part driven by its use in patients who may not meet the originally intended criteria.
In addition, the widespread utilization of TCAR has also broadened the application of stenting in the carotid artery to clinical scenarios where it historically did not offer therapeutic equipoise to CEA.28–30 The TCAR stenting technique was approved by the FDA in part due to it being “substantially equivalent” to other stenting procedures (e.g., TF-CAS).14 As such, it would seem that its routine use in symptomatic patients, particularly those who have suffered a stroke, would be tempered by past randomized trial data documenting higher periprocedural stroke risk for TF-CAS versus CEA.28–30 However, our findings suggest the opposite. In fact, the percent of patients who underwent TCAR in the setting of stroke increased over the study interval, to nearly one-third of patients in 2021. In addition, our propensity score model suggests that TCAR patients are being selected from a pool of patients, some of whom would have likely undergone CEA, while others would have undergone TF-CAS, rather than replacing one specific procedure. CEA has well-documented low periprocedural stroke or death risks, and well-known favorable long-term durability and low restenosis rates, with several randomized controlled trials comparing it with both medical intervention and TF-CAS.19, 28, 29, 31–37 Furthermore, TCAR is associated with substantially increased cost when compared to CEA, both in the periprocedural period, and per quality-adjusted life year.38, 39 As such, it is somewhat surprising to see CEA market share being replaced by TCAR, particularly as a first-line option in the peri-stroke period. Taken together, our results document that the introduction of TCAR has led to a paradigm shift in the contemporary management of carotid artery stenosis, with TCAR now established as a mainstay of clinical practice.
It is worth highlighting that the rapid adoption and broad diffusion of TCAR documented herein has occurred in the absence of a dedicated randomized clinical trial. Approved procedures for carotid stenosis have traditionally rested on a strong foundation of level 1 evidence. Both CEA and TF-CAS have been the focus of multiple randomized clinical trials documenting their efficacy and long-term durability.19, 28–31, 33, 35–37, 40 In contrast to this precedent, to date, no randomized trial comparing TCAR to CEA, TF-CAS, or medical therapy has been completed or is enrolling. Furthermore, TCAR’s long-term durability still remains unknown, as currently, 1-year outcome estimates are the longest follow-up available among large observational studies.10, 11, 27, 41, 42 Despite this, in May of 2022, the FDA granted an expanded indication to TCAR, approving its use among standard-risk patients.43 This widespread utilization and now expanded FDA-approval highlights the need for a rigorous randomized trial to determine the safety and efficacy profile of TCAR versus other treatment modalities, which cannot be established with registry studies alone. However, given TCARs rapid adoption and uptake highlighted herein, a dedicated randomized trial may be unlikely. This underscores the important role that high-quality registry and claims-based analyses will play in informing TCAR’s rightful role in the treatment of both asymptomatic and symptomatic carotid occlusive disease. In addition, it raises important unanswered questions regarding where the balance should lie between regulatory oversight to ensure safe and effective care for patients and fostering medical innovation to advance clinical practice.
Our study has several strengths. We have documented the evolution in baseline characteristics of patients who undergo TCAR, and more specifically of its diffusion to a broader population of recipients among patients suffering from carotid-artery disease, highlighting important differences not previously described.16 There is not consensus in how “high-risk” is defined, with variation across studies.5, 19, 20, 27–29, 44 We included only CMS-approved criteria for our calculations of high risk. In addition, since centers who report TCAR procedures are not simultaneously required to report CEA procedures, measurements of procedure counts may yield biased results. Our method of calculating TCAR uptake in a subanalysis of centers reporting all three carotid revascularization procedures decreases this risk of overestimation and likely provides a more representative estimate of the true use of TCAR among centers adopting it. For data reporting and quality reasons (e.g., the linkage to get region information) we focused on centers that adopted TCAR. Our study is nonetheless significant for being among the first to document the rapid adoption and dissemination of TCAR across the United States and reveals unique insights about the diffusion of TCAR at centers willing to adopt it.
Despite these important findings, this study has several limitations. VQI procedure reporting is voluntary, and therefore is subject to reporting bias. TCAR reporting may be less subject to this bias because Medicare reimbursement for TCAR requires that patients participate in a registry of clinical study. This requirement provides an unusual opportunity to document trends on almost all patients undergoing TCAR, strengthening the inferences from this project. Our propensity score overlap coefficients quantify the proportions of patients who underwent TCAR that are similar to those who underwent CEA and TF-CAS. It does not describe the absolute number (or market share) of patients who may otherwise have undergone CEA or TF-CAS had TCAR not been available. These counterfactual calculations rely in part on the Independence of Irrelevant Alternatives assumption.24–26 We believe that this assumption holds, especially given that the C-statistic discriminating between CEA and TF-CAS in our propensity score models varied minimally from before to after the introduction of TCAR. We are not able to determine the cause of the dramatic uptake of TCAR since its introduction, but it is likely multifactorial, including factors such as reimbursement, physician skillset, and patient preference, among others. Although 56% of VQI participating physicians are non-vascular surgeons (Figure S10), we are not able to compare the distribution of physician specialties performing TCAR per VQI policy to maintain confidentiality.45 For similar reasons we are not able to comment on years of practice and its possible association with procedure choice.
Conclusions:
TCAR has been rapidly adopted and incorporated into real world practice across the United States. It has been performed at 606 centers with over 31,000 implants as of March 2022, representing a 74-fold increase in annual procedure volume from 2016 to 2021. Furthermore, TCAR is now commonly being offered as a first line therapeutic option, even in the setting of symptomatic disease, and among standard-risk patients. Interestingly, this dramatic and rapid therapeutic paradigm shift in carotid revascularization has transpired in the absence of any level 1 evidence or any observational data documenting TCAR’s long-term durability. Given this, registry and claims-based analyses will be essential in measuring TCAR’s safety and effectiveness over time to establish the rightful evidence-based role of TCAR in the treatment of carotid occlusive disease. Moreover, TCAR’s pathway to becoming a mainstay of contemporary practice highlights important unanswered questions surrounding how new medical innovation should be implemented in the United States.
Supplementary Material
What is new?
TCAR has assumed a growing role in carotid intervention and was in use at 606 centers with 31,447 implants as of March 2022, despite the lack of a randomized trial comparing TCAR against CEA, TF-CAS, or medical therapy.
TCAR now represents nearly 1-in-4 carotid interventions at centers reporting all three procedures and is now being performed as a first-line procedural option among patients after ischemic stroke.
Patients who undergo TCAR appear to be clinically similar to those who get CEA or TF-CAS, indicating that TCAR is being preferentially selected by clinicians in place of both procedures.
What are the clinical implications?
The introduction of TCAR has led to a paradigm shift in the contemporary management of carotid artery stenosis, with TCAR now established as a mainstay of clinical practice.
The widespread uptake of TCAR in the absence of level 1 data to support its use raises important unanswered questions regarding where the balance should lie between regulatory oversight to ensure safe and effective care for patients and fostering medical innovation to advance clinical practice.
Funding:
This work was supported in part by the Hitchcock Foundation. All statements in this paper, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Hitchcock-Foundation. The funders had no role in the design or execution of the study.
Abbreviations:
- TCAR
transcarotid artery revascularization
- CEA
carotid endarterectomy
- TF-CAS
transfemoral carotid artery stenting
- VQI
Vascular Quality Initiative
- P
P-value
Footnotes
Disclosures:
The authors have no conflicts of interest to report.
References:
- 1.Ahmad FB and Anderson RN. The Leading Causes of Death in the US for 2020. Jama. 2021;325:1829–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole TS, Mezher AW, Catapano JS, Godzik J, Baranoski JF, Nakaji P, Albuquerque FC, Lawton MT, Little AS and Ducruet AF. Nationwide Trends in Carotid Endarterectomy and Carotid Artery Stenting in the Post-CREST Era. Stroke. 2020;51:579–587. [DOI] [PubMed] [Google Scholar]
- 3.Kumins NH, King AH, Ambani RN, Thomas JP, Kim AH, Augustin G, Wong VL, Harth KC, Cho JS, Colvard B and Kashyap VS. Anatomic criteria in the selection of treatment modality for atherosclerotic carotid artery disease. Journal of vascular surgery. 2020;72:1395–1404. [DOI] [PubMed] [Google Scholar]
- 4.Muller MD, Lyrer P, Brown MM and Bonati LH. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. The Cochrane database of systematic reviews. 2020;2:CD000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Medicare and Medicaid Services. Carotid Artery Stenting Decision Summary. Accessed June 1st, 2022. Available at: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&NCAId=157.
- 6.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ, American College of Cardiology F, American Stroke A, American Association of Neurological S, American College of R, American Society of N, Congress of Neurological S, Society of Atherosclerosis I, Prevention, Society for Cardiovascular A, Interventions, Society of Interventional R, Society of NeuroInterventional S, Society for Vascular M and Society for Vascular S. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. [DOI] [PubMed] [Google Scholar]
- 7.Kwolek CJ, Jaff MR, Leal JI, Hopkins LN, Shah RM, Hanover TM, Macdonald S and Cambria RP. Results of the ROADSTER multicenter trial of transcarotid stenting with dynamic flow reversal. Journal of vascular surgery. 2015;62:1227–34. [DOI] [PubMed] [Google Scholar]
- 8.Malas MB, Leal J, Kashyap V, Cambria RP, Kwolek CJ and Criado E. Technical aspects of transcarotid artery revascularization using the ENROUTE transcarotid neuroprotection and stent system. Journal of vascular surgery. 2017;65:916–920. [DOI] [PubMed] [Google Scholar]
- 9.King AH, Kumins NH, Foteh MI, Jim J, Apple JM and Kashyap VS. The learning curve of transcarotid artery revascularization. Journal of vascular surgery. 2019;70:516–521. [DOI] [PubMed] [Google Scholar]
- 10.Schermerhorn ML, Liang P, Eldrup-Jorgensen J, Cronenwett JL, Nolan BW, Kashyap VS, Wang GJ, Motaganahalli RL and Malas MB. Association of Transcarotid Artery Revascularization vs Transfemoral Carotid Artery Stenting With Stroke or Death Among Patients With Carotid Artery Stenosis. Jama. 2019;322:2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malas MB, Dakour-Aridi H, Kashyap VS, Eldrup-Jorgensen J, Wang GJ, Motaganahalli RL, Cronenwett JL and Schermerhorn ML. TransCarotid Revascularization with Dynamic Flow reversal versus Carotid Endarterectomy in the Vascular Quality Initiative Surveillance Project. Ann Surg. 2022. Aug 1;276(2):398–403. doi: 10.1097/SLA.0000000000004496. Epub 2020 Sep 15. [DOI] [PubMed] [Google Scholar]
- 12.Columbo JA, Martinez-Camblor P, Stone DH, Goodney PP and O’Malley AJ. Procedural Safety Comparison Between Transcarotid Artery Revascularization, Carotid Endarterectomy, and Carotid Stenting: Perioperative and 1-Year Rates of Stroke or Death. Journal of the American Heart Association. 2022;11:e024964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Columbo JA, Martinez-Camblor P, O’Malley AJ, Stone DH, Kashyap VS, Powell RJ, Schermerhorn ML, Malas M, Nolan BW and Goodney PP. Association of Adoption of Transcarotid Artery Revascularization With Center-Level Perioperative Outcomes. JAMA Netw Open. 2021;4:e2037885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. 510(k) Submission Programs. Accessed June 1st, 2022. Available at: https://www.fda.gov/medical-devices/premarket-notification-510k/510k-submission-programs.
- 15.AbuRahma AF, Avgerinos ED, Chang RW, Darling RC 3rd, Duncan AA, Forbes TL, Malas MB, Perler BA, Powell RJ, Rockman CB and Zhou W. The Society for Vascular Surgery implementation document for management of extracranial cerebrovascular disease. Journal of vascular surgery. 2022;75:26S–98S. [DOI] [PubMed] [Google Scholar]
- 16.Stonko DP, Goldsborough E 3rd, Kibrik P,Zhang G, Holscher CMand Hicks CW . Use of Transcarotid Artery Revascularization, Transfemoral Carotid Artery Stenting, and Carotid Endarterectomy in the US From 2015 to 2019. JAMA Netw Open. 2022;5:e2231944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medical SilkRoad. TCAR Surveillance Project. Accessed January 1st, 2022. Available at: https://silkroadmed.com/tcar-surveillance-project/. [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Carotid Artery Stenting (CAS) Investigational Studies. Accessed December 1st, 2022. Available at: https://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/Carotid-Artery-Stenting-CAS-Investigational-Studies.
- 19.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, Popma JJ, Snead DB, Cutlip DE, Firth BG, Ouriel K, Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy I. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. [DOI] [PubMed] [Google Scholar]
- 20.Hopkins LN, Myla S, Grube E, Wehman JC, Levy EI, Bersin RM, Joye JD, Allocco DJ, Kelley L and Baim DS. Carotid artery revascularization in high surgical risk patients with the NexStent and the Filterwire EX/EZ: 1-year results in the CABERNET trial. Catheter Cardiovasc Interv. 2008;71:950–60. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Camblor P About the use of the overlap coefficient in the binary classification context. Communications in Statistics - Theory and Methods. 2022:1–11 Accessed January 1, 2023, Available at: 10.1080/03610926.2022.2032754. [DOI] [Google Scholar]
- 22.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Camblor P, De Uña-Álvarez J and Corral N. k-Sample test based on the common area of kernel density estimators. Journal of Statistical Planning and Inference. 2008;138:4006–4020. [Google Scholar]
- 24.Ray P Independence of Irrelevant Alternatives. Econometrica. 1973;41:987–991. [Google Scholar]
- 25.Hausman J and McFadden D. Specification Tests for the Multinomial Logit Model. Econometrica. 1984;52:1219–1240. [Google Scholar]
- 26.Small K and Hsiao C. Multinomial Logit Specification Tests. International Economic Review. 1985;26:619–27. [Google Scholar]
- 27.Kashyap VS, Schneider PA, Foteh M, Motaganahalli R, Shah R, Eckstein HH, Henao S, LaMuraglia G, Stoner MC, Melton J, Massop D, Hanover T, Titus J, Moore WS, Rodriguez-Carvajal R, Malas MB, Arko FR 3rd, Pierce D, Anain P, Oskin T and Investigators* R. Early Outcomes in the ROADSTER 2 Study of Transcarotid Artery Revascularization in Patients With Significant Carotid Artery Disease. Stroke. 2020;51:2620–2629. [DOI] [PubMed] [Google Scholar]
- 28.Group SC, Ringleb PA, Allenberg J, Bruckmann H, Eckstein HH, Fraedrich G, Hartmann M, Hennerici M, Jansen O, Klein G, Kunze A, Marx P, Niederkorn K, Schmiedt W, Solymosi L, Stingele R, Zeumer H and Hacke W. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–47. [DOI] [PubMed] [Google Scholar]
- 29.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, Larrue V, Lievre M, Leys D, Bonneville JF, Watelet J, Pruvo JP, Albucher JF, Viguier A, Piquet P, Garnier P, Viader F, Touze E, Giroud M, Hosseini H, Pillet JC, Favrole P, Neau JP, Ducrocq X and Investigators E-S. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. [DOI] [PubMed] [Google Scholar]
- 30.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lal BK, Meschia JF and Investigators C. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobson RW 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JBand Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–7. [DOI] [PubMed] [Google Scholar]
- 32.Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Jama. 1995;273:1421–8. [PubMed] [Google Scholar]
- 33.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–87. [PubMed] [Google Scholar]
- 34.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE and Spence JD. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–25. [DOI] [PubMed] [Google Scholar]
- 35.Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D and Asymptomatic Carotid Surgery Trial Collaborative G. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet. 2010;376:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, Moore WS, Hill MD, Mantese VA, Clark WM, Timaran CH, Heck D, Leimgruber PP, Sheffet AJ, Howard VJ, Chaturvedi S, Lal BK, Voeks JH, Hobson RW, 2nd and Investigators C. Long-Term Results of Stenting versus Endarterectomy for Carotid-Artery Stenosis. N Engl J Med. 2016;374:1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenfield K, Matsumura JS, Chaturvedi S, Riles T, Ansel GM, Metzger DC, Wechsler L, Jaff MR, Gray W and Investigators AI. Randomized Trial of Stent versus Surgery for Asymptomatic Carotid Stenosis. N Engl J Med. 2016;374:1011–20. [DOI] [PubMed] [Google Scholar]
- 38.Cui C, Ramakrishnan G, Murphy J and Malas MB. Cost-effectiveness of transcarotid artery revascularization versus carotid endarterectomy. Journal of vascular surgery. 2021;74:1910–1918 e3. [DOI] [PubMed] [Google Scholar]
- 39.Sridharan ND, Chaer RA, Smith K and Eslami MH. Carotid endarterectomy remains cost-effective for the surgical management of carotid stenosis. Journal of vascular surgery. 2022;75:1304–1310. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC and Barnett HJ. The North American Symptomatic Carotid Endarterectomy Trial : surgical results in 1415 patients. Stroke. 1999;30:1751–8. [DOI] [PubMed] [Google Scholar]
- 41.Kashyap VS, So KL, Schneider PA, Rathore R, Pham T, Motaganahalli RL, Massop DW, Foteh MI, Eckstein HH, Jim J, Leal Lorenzo JI and Melton JG. One-Year Outcomes After Transcarotid Artery Revascularization (TCAR) in the ROADSTER 2 trial. J Vasc Surg. 2022. Aug;76(2):466–473.e1. doi: 10.1016/j.jvs.2022.03.872. Epub 2022 Apr 2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GQ, Bose S, Stonko DP, Abularrage CJ, Zarkowsky DS and Hicks CW. Transcarotid artery revascularization is associated with similar outcomes to carotid endarterectomy regardless of patient risk status. J Vasc Surg. 2022. Aug;76(2):474–481.e3. doi: 10.1016/j.jvs.2022.03.860. Epub 2022 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Food and Drug Administration. Premarket approval for Transcarotid Artery Revascularization (TCAR) in Standard-Risk Patients. Accessed December 1, 2022. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P140026S016.
- 44.International Carotid Stenting Study i, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ and Brown MM. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Society for Vascular Surgery Vascular Quality I. SVS PSO Data Analysis Guidelines for Use. 2022;2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


